NMS candidate enrolment
This topic
tells you about candidate enrolment to theNew
medicine service(NMS).
You can register a patient for NMS at the time of printing the labels for the medication.
Alternatively, you can register a patient's consent from the patient medication history when they have initially not consented to NMS.
![]() Only qualifying items prescribed on NHS
prescriptions will alert to potential NMS candidacy for a patient. A qualifying
item dispensed from a private prescription will not generate NMS alerts.
Only qualifying items prescribed on NHS
prescriptions will alert to potential NMS candidacy for a patient. A qualifying
item dispensed from a private prescription will not generate NMS alerts.
Display on screen alert
When the NMS configuration is set to Display on screen alert and you dispense a prescription which includes one or more medication items that meet NMS criteria, then the system will generate a screen prompt when completing the prescription.
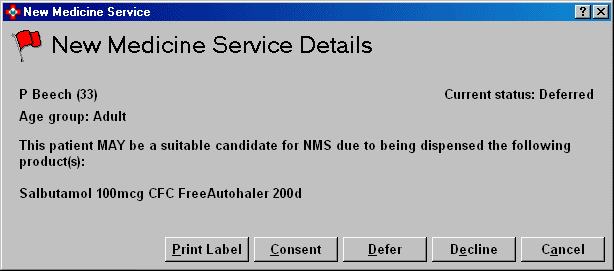
Print label
If the patient is a candidate for NMS but is not present to collect their medication - and be asked about enrolment - you can produce an additional label as a reminder for when they return to collect their medication.
-
Click the Print label button. Alternatively, press
 &
& 
-
A label prints out identifying the patient and the qualifying prescription item(s).

If the patient is a candidate consents for NMS - perhaps you identify them as a candidate before any labels are printed - they complete the required consent form. When labelling is complete, you can then record their consent.
-
Click the Consent button. Alternatively, press
 &
& 
From 1st October 2011, written consent must be obtained when providing services such as NMS & MURs. Verbal consent is insufficient. See these two consent form examples from the PSNC website for NMS consent form templates you can use in bothPDF format and MS Word format.
If the you are not in a position to obtain patient consent but the patient has not declined to participate - they may wish to consider their candidacy before making a decision - you can record a deferred decision.
-
Click the Defer button. Alternatively, press
 &
& 
If the patient is a candidate for NMS but is not present to collect their medication - and be asked about enrolment - you can produce an additional label as a reminder for when they return to collect their medication.
-
Click the Print label button. Alternatively, press
 &
&

Finally, if you want to close the prompt without selecting
any option, click the Cancel button.
Alternatively, press ![]() &
& ![]()
Advisory box messages
The advisory box on the Dispensary tab indicates medication that falls under the scope of NMS.

Some medication could also be part of another service. E-NMS indicates the New medicine service in England. E-tMUR indicates targeted MURs in England & W-tMUR reflects Wales only. EW-tMUR indicates the product is both part of the English and the Welsh service.
-
Double click on the advisory message.
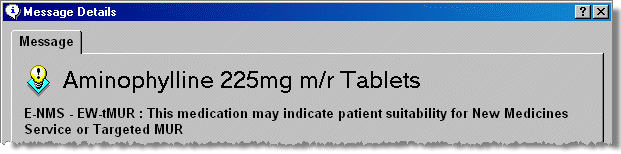
-
The full message appears.
-
Click the Close button to exit from this Window.
Updating decisions
Any patient can change their mind and you can record the new decision. For example, at a later point you can register any the consent of any patient who has declined initially or where you have printed a reminder label.
-
Access the patient history.
The NMS event that has taken place is displayed.
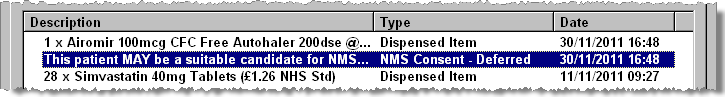
-
Highlight the NMS event you wish to update and double click.
-
The NMS alert appears on screen (see above).
-
Select the new response.
-
The type is updated e.g. an NMS consent change from Deferred to Consented.
![]() Note:
Do not forget to obtain written consent
from the patient.
Note:
Do not forget to obtain written consent
from the patient.
Formulation changes
If the medicine formulation for an NMS qualifying product is changing, subsequent alert will appear.
Such a patient might not normally be eligible for NMS - they may have enrolled on the service for their original medication - but they may now require additional guidance for the new formulation. For example, they may be changing from tablets to an inhaler and would require guidance on the correct use of the inhaler.
Some formulation changes will not be sufficient to activate a further prompt e.g. a change from tablets to capsules.
Updated 1st August 2013