Endorsing Specials
The special items endorsement selection will only be visible if the PRA is set to England or Wales.
Part VIIIB
If you have the Special items setting in Pharmacy Details - Dispensing - Endorsing set the system to Always make me choose, select the SP or ED endorsement according to how your specials supplier has manufactured the product:
-
SP - if they have a MHRA licence or,
-
ED - if manufacture has taken place under a section 10 exemption.
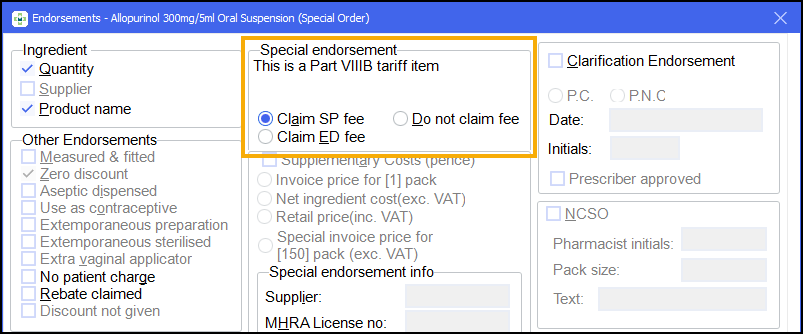
When the specials endorsement option is selected, the dispensed quantity and product name options are also automatically selected. However, these additional endorsements can be removed. See Removing endorsements for details.
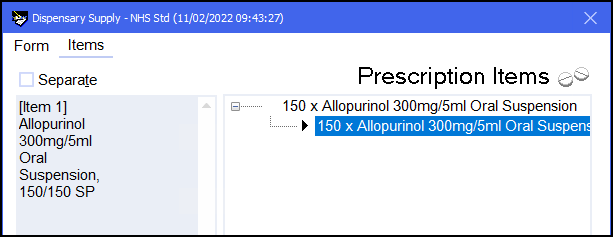
The completed endorsement will appear in the left hand side of the Dispensary Supply screen:
Non-part VIIIB
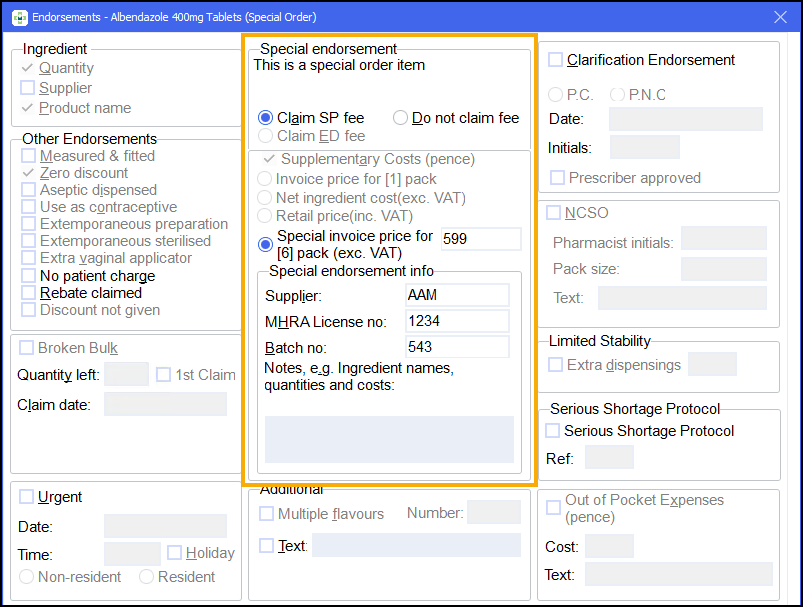
There are some additional details that are required for non-part VIIIB items.
You may have already input these details whilst dispensing, in which case the invoice price, manufacturer’s MHRA license number and batch number displays on the Endorsements screen.
If the details have not been input already, these fields will become enabled when Claim SP fee is selected and the system detects that the item is not in the Part VIIIB tariff:
-
Enter the following details:
-
Invoice price, excluding VAT. This should be entered as pence without any decimal point.
-
Supplier name.
-
MHRA license number.
-
Item's batch number.
-
-
When all details are complete, select OK
 .
.
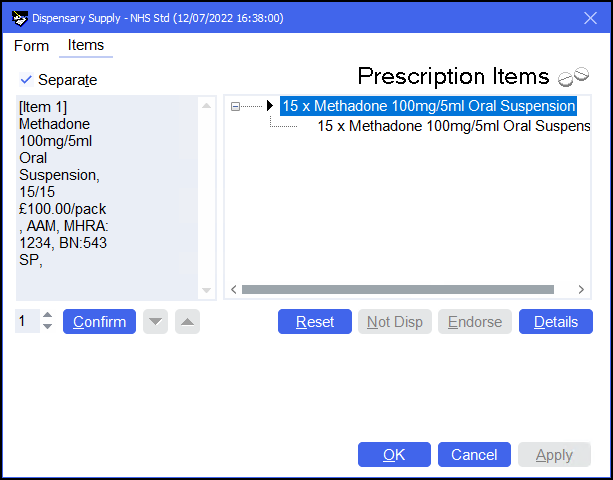
The completed endorsement will appear in the left hand side-bar.
Removing endorsements
Pharmacy Manager errs on the side of caution with endorsements to minimise losses in remuneration that could be occurred with some prescriptions. Consequently, endorsements that are strictly not needed can be removed.
For example, the Quantity and Product name endorsements can be unchecked for a Part VIIIB special in order to reduce the space taken in the left hand side-bar:
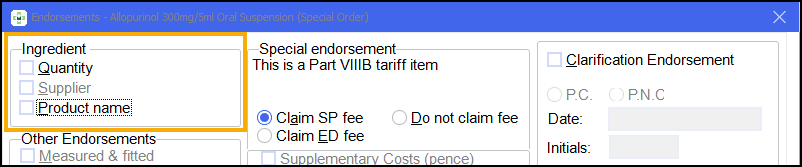
If you do this, the system prompts you to follow that rule for the Product namefor all future prescriptions:
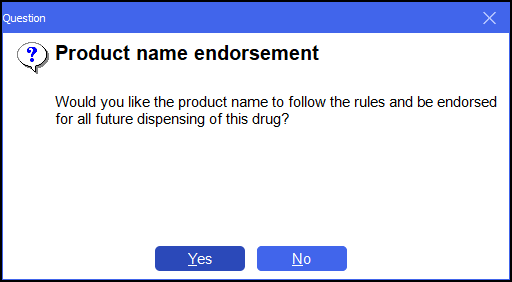
Answer Yes or No as preferred. The result would be an SP endorsement only, which is sufficient in this instance.
Extemporaneous Dispensing
For either third party items or items manufactured in the Pharmacy under section 10, there is further endorsement information to notify of each ingredient used. This information is also required for non-Part VIIIB ED endorsements if broken bulk is to be claimed for the ingredients.
Pharmacy Manager does not keep a database of ingredients and so cannot manage this process. However, in this scenario, a free type text box will be offered for you to manually enter this information .
On the Endorsements screen, select Claim ED fee:
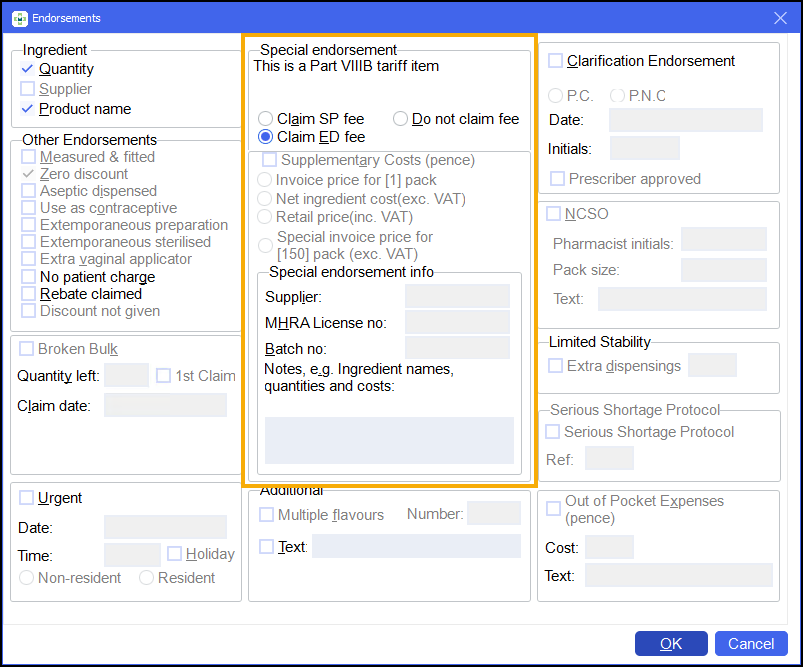
-
Enter the following details:
-
Invoice price, excluding VAT. This should be entered as pence without any decimal point.
-
Supplier name.
-
MHRA license number.
-
Item's batch number.
-
Ingredients.
-
-
When all details are complete, select OK
 .
.
The free type edit box for the ingredients will only be visible if ED endorsing is selected and the item is not part of an EPSR2 prescription. Pharmacy Manager does not govern the format of the text but will limit the number of characters to ensure it can fit on a paper printout.
An example could be:
Aqueous cream,198g,123p
Sulphur,2g,10p
The additional information will be written to the database when entered to ensure that records are kept.
Electronic claims
For sites in England that are EPSR2 configured, claims for Part VIIIB specials must be made electronically. Consequently, endorsements should be made to ensure correct remuneration.