Introduction to FGM Recording and Reporting (2015/16)
The Vision+ FGM Recording and Reporting (2015/16) pathway enables you to accurately view and record a female patient's Female Genital Mutilation (FGM) history. The Practice Report generated in Vision+ helps decide whether a patient is appropriate to include in the Excel claim file that can be uploaded, via CAP (Clinical Audit Platform), to NHS England:
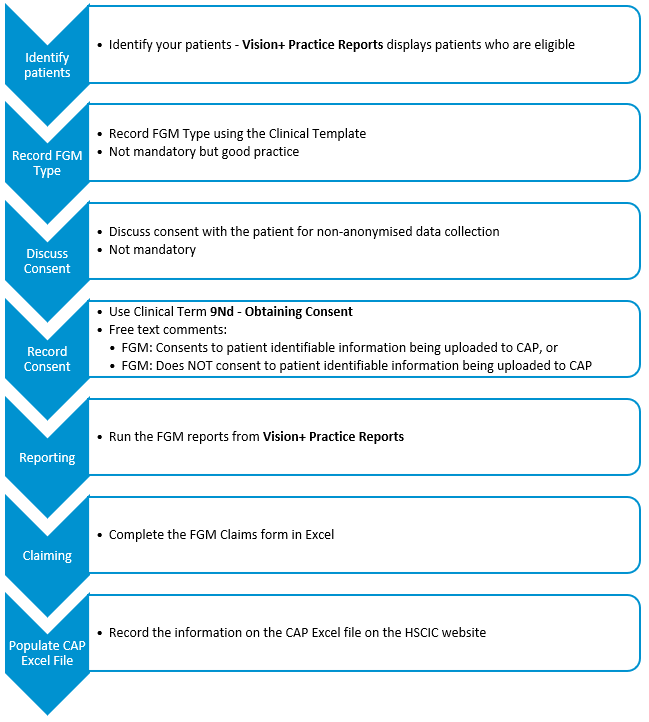
To download the FGM Recording and Reporting (2015/16) pathway, see Downloading Web Files.
Patient Inclusion/Exclusion Criteria
The following criteria apply:
- Male patients are automatically excluded.
- Female patients of all ages are eligible.
- Female patients with FGM clinical terms automatically list in the report. The clinical terms included are:
- 15K - History of Female Genital Mutilation
- K578 - Female Genital Mutilation
- K5780 - FGM type I - WHO classification
- K5781 - FGM type II - WHO classification
- K5782 - FGM type III - WHO classification
- K5783 - FGM type IV - WHO classification
- Consent is not mandatory, but it is recommended, therefore, the FGM reports display the different consent statuses per patient (consent/dissent/absent) but the final decision to include a patient or not is with the practice.
- GP2GP does not pick up the specific free-text around the issue of consent, so new patients to the practice with FGM clinical terms are reported by the module as having consent absent with relation to the extract.Note - For more information on the FGM Prevention Programme, see FGM Prevention Programme (PDF). For the WHO FGM definitions, see WHO definitions.