Endorsing Specials and Imports - England
The way in which unlicensed specials and imports are reimbursed changed on 1st November 2011.
Some unlicensed specials and their new reimbursement prices are to be included in the Drug Tariff in a new section called Part VIIIB. The requirements for endorsing unlicensed specials and imports vary depending on whether the product is listed in Part VIIIB or not and how the product was sourced.
Below are examples of different situations and how the products should be endorsed. Pharmacy Manager supports the application of the various endorsing options with appropriate alerts for endorsements for both paper and electronic prescriptions.
Product listed in Part VIIIB
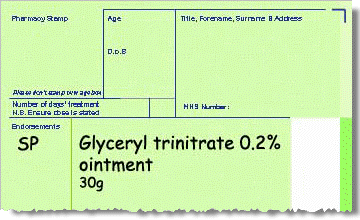
-
SP should be endorsed to claim the agreed fixed fee for sourcing the product from the supplier.
-
Alternatively, endorse ED to claim the agreed fixed fee for extemporaneous dispensing, if the product has been extemporaneously dispensed by the contractor or a third party.
-
No price endorsement needed.
Product not listed in Part VIIIB (not imports)
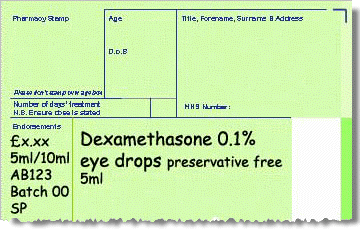
-
Invoice price of product minus any discount/rebates.
-
Pack size used to dispense from.
-
Manufacturer’s MHRA specials licence number.
-
Batch number of unlicensed special.
-
SP should be endorsed to claim the agreed fixed fee for sourcing the product from the supplier.
-
Alternatively, endorse ED to claim the agreed fixed fee for extemporaneous dispensing, if the product has been extemporaneously dispensed by the contractor or a third party.
Product not listed in Part VIIIB (import)
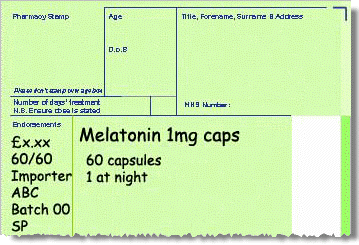
-
Invoice price of product minus any discount/rebates.
-
Pack size used to dispense from.
-
Importer’s MHRA licence number.
-
Batch number of imported medicine (if available).
-
SP should be endorsed to claim the agreed fixed fee for sourcing the product from the supplier.
Extemporaneously Dispensing
Where a special, not listed in Part VIIIB, has been prepared under the manufacturing part of the Section 10 exemption from the Medicines Act 1968, either by the contractor or by a third party, the contractor must endorse:
-
The names, quantities and cost of the ingredients used in preparing the special.
-
ED to claim the agreed fixed fee for extemporaneously dispensing the product.
Broken bulk cannot be claimed for an unlicensed special or import. It can only be claimed on the ingredients used when a product is extemporaneously dispensed.