|
|
Creating a Yellow Card Report via the Drug Allergy and Intolerance Screen
To create a Yellow Card report from the Drug Allergy and Intolerance - Add screen:
- From Consultation Manager, select the patient and make sure a consultation is started.
- Go to Add - Drug Allergy/Adverse Reaction.
- Complete the Add Drug Allergy and Intolerance - Add form details as usual:
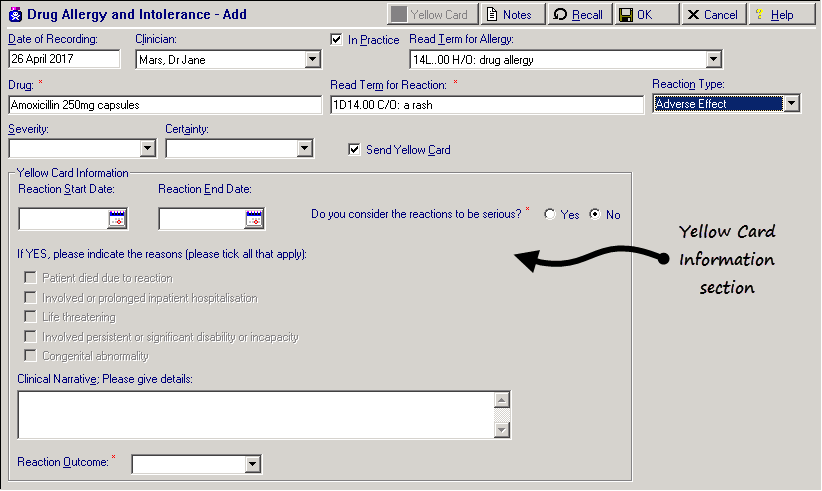
Remember - You must enter drug, reaction type, severity and certainty details when adding a drug allergy/intolerance.
- Tick Send Yellow Card (Alt+C)
 . The Yellow Card Information form is now active and can be completed:
. The Yellow Card Information form is now active and can be completed: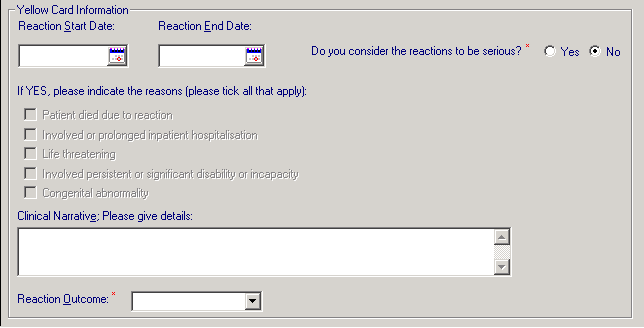
- Reaction Start/End Date - Enter the dates or choose the dates from the calendar buttons. An End Date should be entered if you are selecting a Reaction Outcome of Recovered
- Do you consider the reactions to be serious? - This is a mandatory entry. Select Yes or No:
- If you select Yes, If YES, please indicate the reasons (tick all that apply) displays - Select one of the following:
- Patient died due to reaction
- Involved prolonged inpatient hospitalisation
- Life threatening
- Involved persistent or significant disability or incapacity
- Congenital abnormality
- If you select No, continue on to the next section.
- Clinical Narrative; Please give details: - This is a free text box to enter other medically significant details. Please make sure that you do not enter any patient identifiable information. This is transferred to the report.
- Reaction Outcome - This is a mandatory entry. Options include:
- Recovered
- Recovering - If you select this option, the Reaction End Date should be left blank
- Not recovered
- Recovered with sequelae
- Fatal
- Unknown
- If the Reaction Outcome is set as Recovering leave the Reaction End Date blank
- Now, select Yellow Card
 .
.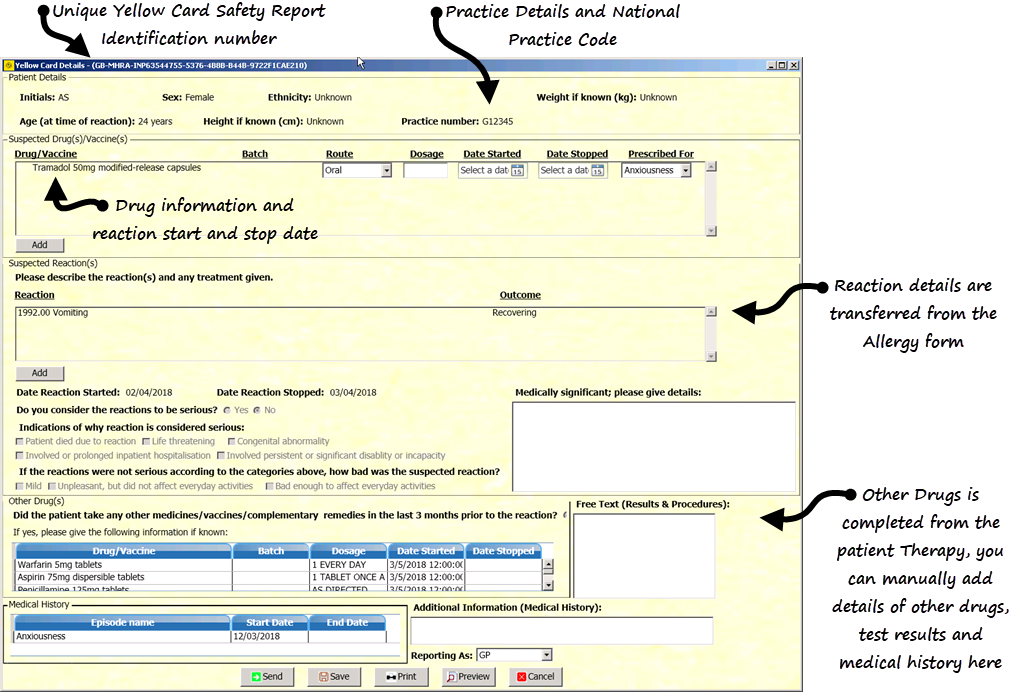
The Yellow Card Details screen is allocated a safety report identification number and is populated with the following information from the patient record:
- Practice Number - Your practice's national identifier.
- Patient Details:
- Patient initials
- Sex
- Ethnicity
- Weight
- Age at the time of reaction
- Height
- Suspected Drug(s)/Vaccine(s) - The drug name and form of the drug entered in the Allergy and Intolerance screen is entered automatically. You can add the following details if required:
- Batch number
- Route
- Dosage
- Date Started
- Date Stopped
- Prescribed for - This contains a list of medical history entries from the last 12 months to select from.
- Add - Select to add additional medication if required.
 Important - If you suspect the adverse reaction may be due to more than one medication then this can be added as an additional suspect drug. If you suspect a different medication of causing a separate reaction, please report this via a separate Yellow Card.
Important - If you suspect the adverse reaction may be due to more than one medication then this can be added as an additional suspect drug. If you suspect a different medication of causing a separate reaction, please report this via a separate Yellow Card.
- Suspected Reaction(s) - The Reaction Read code and other details of severity entered in the Allergy and Intolerance screen is entered automatically. Additional suspected reactions can be added here.
If you selected Yes to the 'Do you consider the reactions serious' section you must select an option from either the:
- Indications of why reaction is considered serious section, or
- If the reactions were not serious according to the categories above, how bad was the suspected reaction? section
- Other Drugs - Medicines, vaccines and any complementary medicines taken in the last 3 months prior to the reaction. This section is completed automatically from the patient record.
- Free Text (Results and Procedures) - Free text can be added here if required to supply additional information to help the MHRA assess the Yellow Card report.
- Medical History - This section is completed automatically from the patient record.
- Additional information (Medical History) - Free text can be added here if required to supply additional information to help the MHRA assess the Yellow Card report. For congenital abnormalities please state all other drugs taken during pregnancy and the date of the last menstrual period. If you are reporting a side effect in a child that occurred as a result of medicines taken by the mother during pregnancy please provide as much information as you can on any previous obstetric history, dates and findings of any ultrasound scans, folate dose and start date, pre conception pregnancy counselling and any drugs taken or stopped during pregnancy. Please also provide details of any delivery complications, birth weight, gestational age and APGAR score for the child.
- Reporting As - Select your role in the practice, the default is GP.
- You can now select Send
 . Other options are:
. Other options are:- Save
 - If you do not want to send the report now, select Save and then OK on the Drug Allergy and Intolerance screen. You are asked if you want to create a task to remind you that the patient has an unsent Yellow Card report which you can process at a future date. See Unsent Yellow Card Reports.
- If you do not want to send the report now, select Save and then OK on the Drug Allergy and Intolerance screen. You are asked if you want to create a task to remind you that the patient has an unsent Yellow Card report which you can process at a future date. See Unsent Yellow Card Reports. - Print
 - Select to print a copy of the report.
- Select to print a copy of the report. - Preview
 - Select to view a copy of the report.
- Select to view a copy of the report. - Cancel
 - Select to discard the report.
- Select to discard the report.
- Save
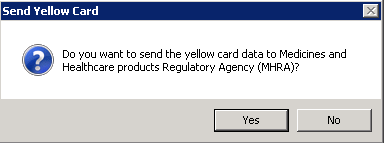
- Select Yes when asked "Do you want to send the yellow card data to Medicines and Healthcare products Regulatory Agency (MHRA)?"
- A progress bar displays whilst the message is sent and a confirmation message displays confirming whether the message has been successfully transmitted.
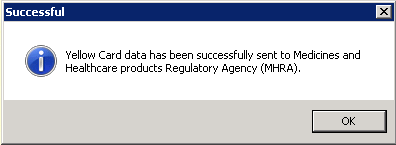
- Select OK to be returned to the Add Allergy/Intolerance screen.
- Select OK
 to save
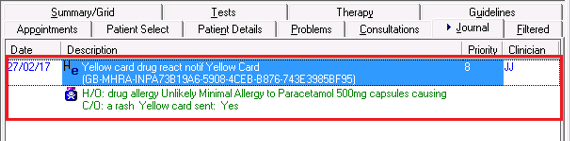
to save - The Yellow Card report is now added to the patient record within the Drug Allergies and Adverse Reactions SDA and a Medical History is recorded with the Read code 9G4..11 Yellow card drug react notif. This ensures the information is transferred as part of the GP2GP process.
Please note the following:
- Yellow Card reports do not contain any patient identifiable information, therefore you do not need to obtain patient consent in order to send a report.
- Yellow Cards can be used for reporting suspected adverse drug reactions to medicines, vaccines, herbal or complementary products, whether self-medicated or prescribed. This includes suspected adverse drug reactions associated with misuse, overdose, medication errors or from use of unlicensed and off-label medicines.
- When inactivating a repeat medication, you are prompted if you would like to create a Yellow Card report. See Creating a Yellow Card Report when Inactivating a Repeat Master.