What's New?
Important please read:
NHS England are now providing weekly updates to the 'At Risk' patient list. On receipt of this list we will add the following Medical History to any patients that newly qualify:
By default the History entry will have:
- Event Date – Date the patient was added to the Shielded Patient List (SPL) as provided by NHS England
- Read Term for Characteristic - 14Or.00 High risk category for developing complication from COVID-19 infection
- Comment – Reason provided by NHS England
- Priority – 3
- End Date – Matches Event Date
They will be added with a Consultation Type of Administration.
These patients have already been added to the national SPL and been contacted by NHS England.
We are currently receiving a weekly updated batch of patients newly identified as 'vulnerable'. NHS Scotland have requested we run this utility on your system with the new batches each week to ensure you have the latest patient information.
NSS are responsible for all communications regarding coronavirus updates for Scotland, please refer to their website for information
NWIS are now providing a regular list of patients that are newly qualified for the 'At Risk' category. On receipt we have been directed to add the following Medical History entry to the patient's record:
- Read Term for Characteristic - 9d44.00 Risk of exposure to communicable disease
- Comment – Central analysis of NHS Data has indicated the patient may be at high risk. Added at NWIS request
- Priority – 1
These entries will have a Consultation Type of Administration.
COVID Spring 2023 Campaign (May 2023)
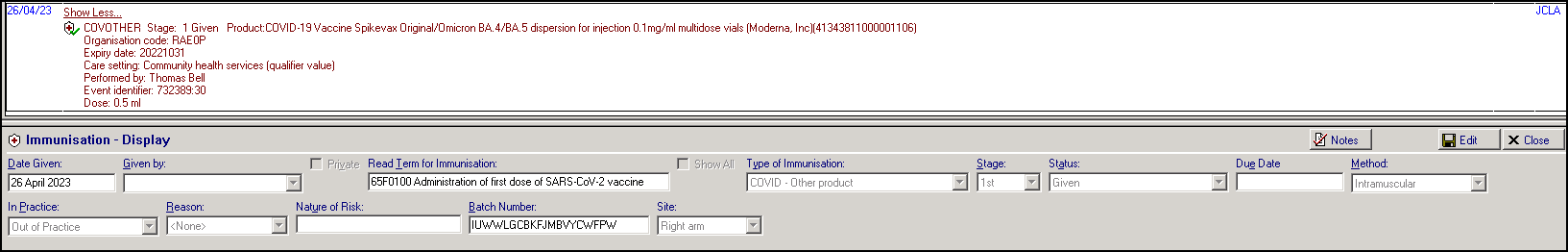
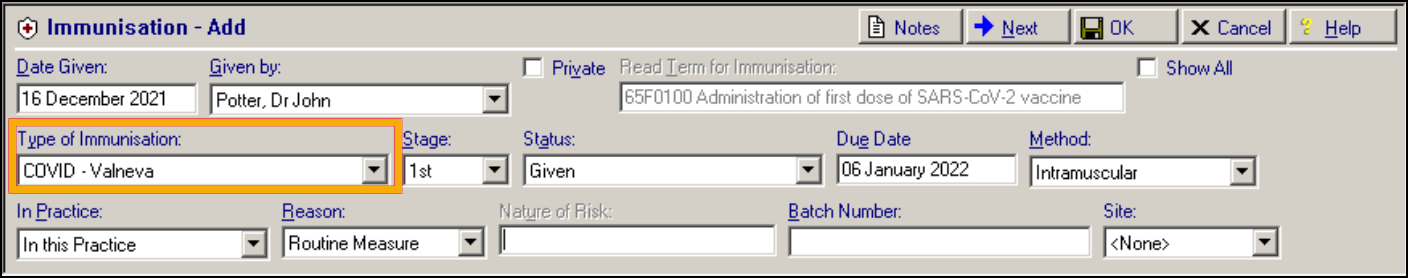
Patients receiving a vaccine during the Spring 2023 campaign, have an immunisation entry added to their patient record, for example:
Immunisation Entry of '65F0100 Administration of first dose of SARS-CoV-2 vaccine':
Previous Releases
Changes have been made to the following COVID vaccine brand names. This is designed to assist you if you only use the product brand name.
|
Immscode |
Current Name |
New Name |
|---|---|---|
|
COVPFIZER |
COVID-19 - Pfizer/BioNTech |
COVID - Pfizer Comirnaty |
|
COVMODERNA |
COVID-19 - Moderna |
COVID - Moderna Spikevax |
|
COVOXFORD |
COVID-19 - Oxford/AstraZeneca |
COVID - AstraZeneca Vaxzevria |
|
COVJANSSEN |
COVID-19 - Janssen |
COVID - Janssen |
|
COVVALNEVA |
COVID-19 - Valneva |
COVID - Valneva |
|
COVNOVAVAX |
COVID-19 - Novavax |
COVID - Novavax |
|
COVMEDICAGO |
COVID-19 - Medicago |
COVID - Medicago |
|
COVOTHER |
COVID-19 - Other product |
COVID - Other product |
The Coronavirus Vaccination Monitoring audit has been updated to identify patients who have received a 3rd Dose and are now eligible for a Booster Dose.
Click here to download the latest Coronavirus Vaccination Clinical Audit.
Click here for instructions on downloading and importing Clinical Audits.
We can now confirm that Moderna Half Dose and Overseas vaccination data from national vaccination systems are being imported and written back to your patient’s record. We will continue to import this data daily.
-
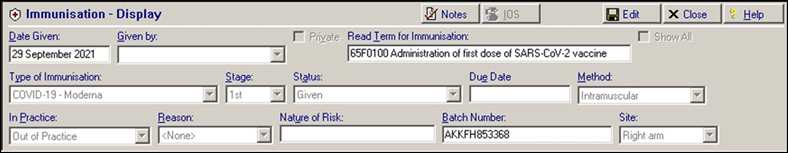
Patients receiving the Moderna Half Dose have an immunisation and a medical history entry added to their patient record, for example:
-
Immunisation Entry of '65F0100 Administration of first dose of SARS-CoV-2 vaccine':

-
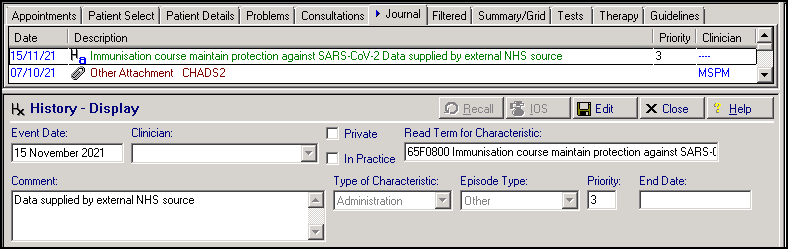
Medical History Entry of '65F0800 Immunisation course to maintain protection against SARS-CoV-2':
-
-
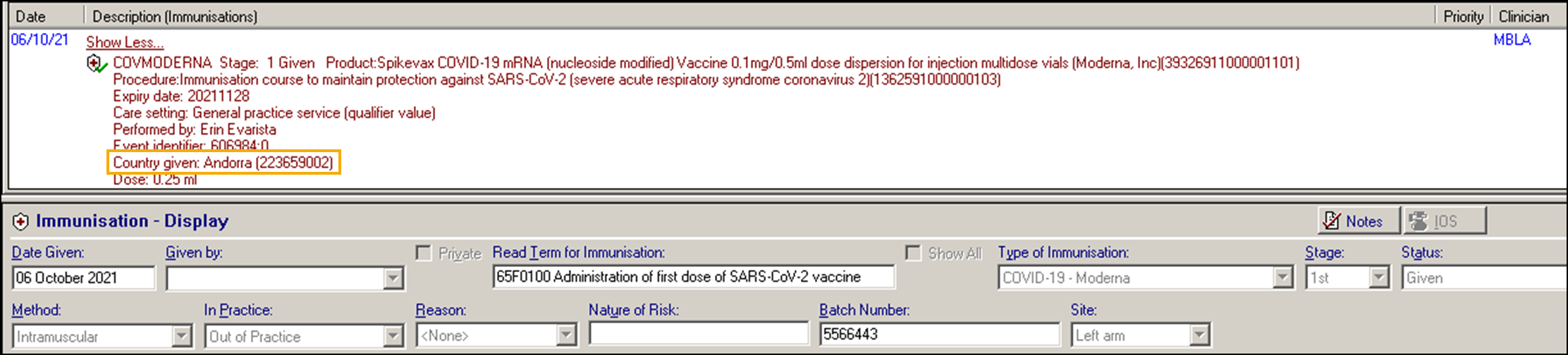
Patients who have received a vaccination Overseas have an immunisation entry automatically added to their patient record with the country where the vaccination was given provided in the notes.
An example of this is an Immunisation Entry of '65F0100 Administration of first dose of SARS-CoV-2 vaccine':
We can now confirm that in addition to the Primary Dose vaccinations Maintenance/Booster Dose, Moderna Half Dose and Third Dose vaccination data from national vaccination systems are being imported and written back to your patient’s record. We have imported the backlog of data and we will continue to import this data daily.
-
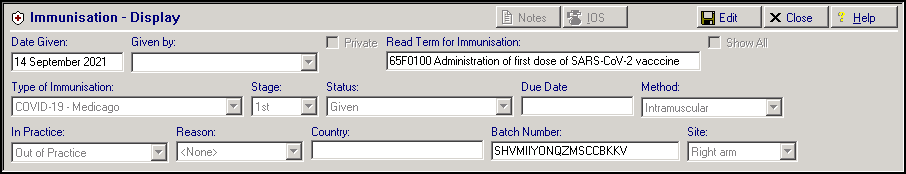
Patients receiving a Maintenance/Booster Dose have an immunisation and a medical history entry automatically added to their patient record, for example:
-
Immunisation Entry of '65F0100 Administration of first dose of SARS-CoV-2 vaccine':
-
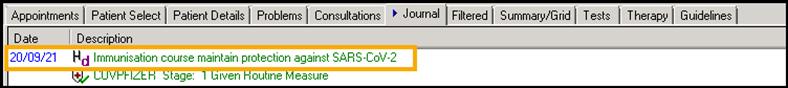
Medical History Entry of '65F0800 Immunisation course to maintain protection against SARS-CoV-2':
-
-
Patients receiving the Moderna Half Dose have an immunisation and a medical history entry added to their patient record, for example:
-
Immunisation Entry of '65F0100 Administration of first dose of SARS-CoV-2 vaccine':


-
Medical History Entry of '65F0800 Immunisation course to maintain protection against SARS-CoV-2':

-
-
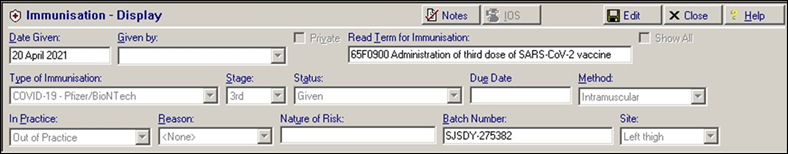
Patients receiving a Third Dose, have an immunisation entry added to their patient record, for example:
Immunisation Entry of '65F0900 Administration of third dose of SARS-CoV-2 vaccine':

As of 15th November 2021 Moderna Half Dose and Third Dose vaccination data from national vaccination systems are imported and written back to your patient's record.
Moderna Half Dose / Third Dose Vaccinations
Moderna Half Dose and Third Dose COVID vaccination data from national vaccination systems is being imported and written back to your patient's record. From the above date, this data is imported daily.
-
Patients receiving the Moderna Half Dose have an immunisation and a medical history entry added to their patient record, for example:
-
Immunisation Entry of '65F0100 Administration of first dose of SARS-CoV-2 vaccine':


-
Medical History Entry of '65F0800 Immunisation course to maintain protection against SARS-CoV-2':

-
-
Patients receiving a Third Dose, have an immunisation entry added to their patient record, for example:
Immunisation Entry of '65F0900 Administration of third dose of SARS-CoV-2 vaccine':


Maintenance/Booster Dose
From 25th October maintenance/booster dose COVID vaccination data from national vaccination systems will be imported and written back to your patient's record. We will continue to import this data daily.
Patients receiving a Maintenance/Booster Dose have an immunisation and a medical history entry automatically added to their patient record, for example:
-
Immunisation Entry of '65F0100 Administration of first dose of SARS-CoV-2 vaccine':

-
Medical History Entry of '65F0800 Immunisation course to maintain protection against SARS-CoV-2':

Please note we have now processed the backlog of maintenance/booster dose COVID vaccination data.
Maintenance/Booster Dose
We have successfully imported the backlog of maintenance/booster dose COVID vaccination data made available to us.
Patients receiving a Maintenance/Booster Dose have an immunisation and a medical history entry automatically added to their patient record, for example:
-
Immunisation Entry of '65F0100 Administration of first dose of SARS-CoV-2 vaccine':

-
Medical History Entry of '65F0800 Immunisation course to maintain protection against SARS-CoV-2':

Daily imports will now run for all primary and maintenance/booster dose COVID vaccination data from today (8th October) in line with NHS Digital's request and recent guidance published by the Joint Committee on Vaccination and Immunisation (JCVI), see JCVI issues updated advice on COVID-19 booster vaccination.
Third Dose Immunosuppressed Vaccination Guidance
The specification for the third dose vaccinations is still being finalised by NHS Digital, in the meantime please see the guidance below:
Maintenance/Booster Dose
We have successfully imported the backlog of maintenance/booster dose COVID vaccination data made available to us, for all practices.
Patients receiving a Maintenance/Booster Dose have an immunisation and a medical history entry automatically added to their patient record, for example:
-
Immunisation Entry of '65F0100 Administration of first dose of SARS-CoV-2 vaccine':

-
Medical History Entry of '65F0800 Immunisation course to maintain protection against SARS-CoV-2':

Daily imports will now run for all primary and maintenance/booster dose COVID vaccination data from today (8th October) in line with NHS Digital's request and recent guidance published by the Joint Committee on Vaccination and Immunisation (JCVI), see JCVI issues updated advice on COVID-19 booster vaccination.
Third Dose Immunosuppressed Vaccination Guidance
The specification for the third dose vaccinations is still being finalised by NHS Digital, in the meantime please see the guidance below:
Maintenance/Booster Dose
On successful completion of Early Live Service, from 11th October we will be importing maintenance/booster dose COVID vaccination data and writing this direct to the patient's record in line with NHS Digital's request and recent guidance published by the Joint Committee on Vaccination and Immunisation (JCVI), see JCVI issues updated advice on COVID-19 booster vaccination.
Patients receiving a Maintenance/Booster Dose have an immunisation and a medical history entry automatically added to their patient record, for example:
-
Immunisation Entry of '65F0100 Administration of first dose of SARS-CoV-2 vaccine':

-
Medical History Entry of '65F0800 Immunisation course to maintain protection against SARS-CoV-2':

Third Dose Immunosuppressed Vaccination Guidance
NHS Digital have provided us with a revised Shielded Patient List (SPL) letter to use when informing your patients they have been added to the SPL.
We have turned this into a Vision template letter, which can be downloaded from here.
NHS Digital have provided us with a revised Shielded Patient List (SPL) letter to use when informing your patients they have been added to the SPL.
We have turned this into a Vision template letter, which can be downloaded from here.
NHS Digital have provided us with an updated Shielded Patient List (SPL) letter to use when informing your patients they are no longer considered high risk.
To facilitate you using this letter when informing your patients they are no longer considered high risk, we have turned this into a Vision 3 template letter, which can be downloaded from here.
NHS Digital have provided us with a revised Shielded Patient List (SPL) letter to use when informing your patients they have been added to the SPL.
We have turned this into a Vision template letter, which can be downloaded from here.
We are pleased to announce you will shortly receive an update to your system allowing the recording of two additional COVID-19 vaccination types from the Immunisation Structured Data Area (SDA):
-
COVID-19 – Novavax
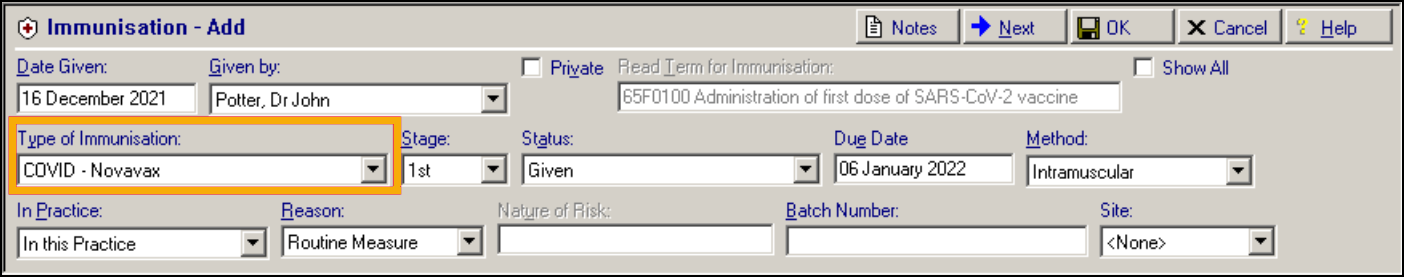
-
COVID-19 – Valneva
NHS Digital have provided us with a revised Shielded Patient List (SPL) letter to use when informing your patients they have been added to the SPL.
We have turned this into a Vision template letter, which can be downloaded from here.
NHS Digital have provided us with a revised Shielded Patient List (SPL) letter to use when informing your patients they have been added to the SPL.
We have turned this into a Vision template letter, which can be downloaded from here.
The letter also includes:
-
Vaccination Guidance for people under the age of 16.
-
Guidance and support available from 1st April.
The Coronavirus Clinical Audits have been updated:
The Coronavirus Vaccination audit file has been updated. As well as the Coronavirus Vaccination Monitoring audit this now includes:
-
COVID-19 Gender and Ethnicity Reporting 2020-21
-
COVID-19 Vaccine Update (GP Patients) 2020-21
Click here to download the latest Coronavirus Vaccination Clinical Audit.
Click here for instructions on downloading and importing Clinical Audits.
We are pleased to announce that we are now ready to start importing historical and daily vaccination data into your patient records.
All historical COVID-19 Vaccination data captured from Pinnacle, the National Immunisation Management System (NIMS), the National Immunisation Vaccination System (NIVS) and the Health and Justice Information Service (HJIS) will be imported and filed to your system starting Saturday 27th March 2021.
Going forward, you will receive new vaccination data on a daily basis.
What do I have to do?
You do not need to do anything. Vaccination data is received and automatically filed to the patient record. NHS Digital is automatically notified if any of the incoming data cannot be matched to a patient record, for example where a patient has transferred out.
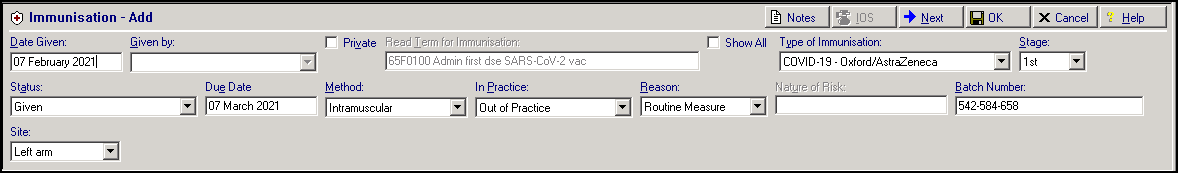
How will my data be filed?
Vaccination data is filed using the current standard COVID-19 vaccination terms (see below). The date on the recording is the event date recorded on the vaccination system and is recorded as “out of practice”. All other relevant fields are populated:
-
65F0100 Administration of first dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure) (SNOMED CT Concept 1324681000000101)
-
65F0200 Administration of second dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure) (SNOMED CT Concept 1324691000000104)
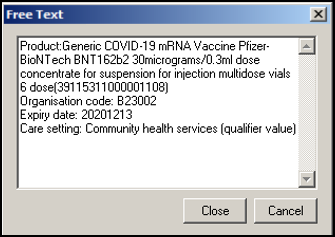
The immunisation notes display a copy of the detailed vaccination details:
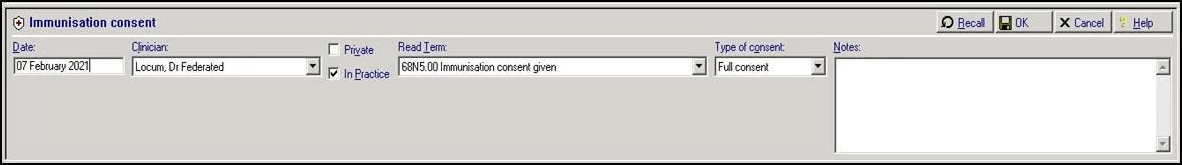
Patient consent data is recorded within the Immunisation Consent Structured Data Area (SDA):
What if I have already entered vaccination data – how are duplicates managed?
Any vaccination data already on the patient record is identified and replaced by the data received from NHS Digital where there is a match to all the key data fields and patient registration status. This only applies to data entered in the Immunisation SDA and does not apply to vaccinations recorded as medical histories or therapy.
What about patients who have dissented?
This data is not being transmitted by NHS Digital. However, explicit consent data is transmitted and is recorded within the Immunisation Consent (SDA) as shown above.
Is adverse reaction data being sent?
At the moment NHS Digital are not sending adverse reaction data, you may, however, receive this data directly from vaccination systems via an email from the central vaccination system.
When can I stop manually recording vaccination data received via email?
As all vaccination data is being routed via NHS Digital via the import files, once the import process commences, you should not manually add the COVID-19 vaccination data received in emails from national systems.
How will I know when we have received new data?
You are not notified when patient records are updated but you can use Clinical Audit results to monitor vaccination data.
Will the patient's Summary Care Record (SCR) be automatically updated with COVID vaccination data?
Yes. SCRs are automatically updated with COVID vaccination data. However, as you are not logged in with a Smartcard when the import process runs, the summaries are queued. You should follow the usual steps for uploading queued summaries to the Spine on a daily basis as detailed in these instructions.
NHS Digital have provided us with a revised Shielded Patient List (SPL) letter to use when informing your patients they have been added to the SPL.
We have turned this into a Vision template letter, which can be downloaded from here.
The letter also includes:
-
Advice and guidance up to the end of March while patients are still shielding.
-
Guidance and support available from 1st April once national shielding is paused.
We are pleased to announce that we are now ready to start importing historical vaccination data into your patient records.
All historical COVID-19 Vaccination data captured from Pinnacle, the National Immunisation Management System (NIMS), the National Immunisation Vaccination System (NIVS) and the Health and Justice Information Service (HJIS) will be imported and filed to your system starting Saturday 27th March 2021.
Going forward, new vaccination data is to be received on a daily basis, we will confirm when this will commence.
What do I have to do?
You do not need to do anything. Vaccination data is received and automatically filed to the patient record. NHS Digital is automatically notified if any of the incoming data cannot be matched to a patient record, for example where a patient has transferred out.
How will my data be filed?
Vaccination data is filed using the current standard COVID-19 vaccination terms (see below). The date on the recording is the event date recorded on the vaccination system and is recorded as “out of practice”. All other relevant fields are populated:

-
65F0100 Administration of first dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure) (SNOMED CT Concept 1324681000000101)
-
65F0200 Administration of second dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure) (SNOMED CT Concept 1324691000000104)
Patient consent data is recorded within the Immunisation Consent Structured Data Area (SDA):

What if I have already entered vaccination data – how are duplicates managed?
Any vaccination data already on the patient record is identified and replaced by the data received from NHS Digital where there is a match to all the key data fields and patient registration status. This only applies to data entered in the Immunisation SDA and does not apply to vaccinations recorded as medical histories or therapy.
What about patients who have dissented?
This data is not being transmitted by NHS Digital. However, explicit consent data is transmitted and is recorded within the Immunisation Consent (SDA) as shown above.
Is adverse reaction data being sent?
At the moment NHS Digital are not sending adverse reaction data, you may, however, receive this data directly from vaccination systems via an email from the central vaccination system.
When can I stop manually recording vaccination data received via email?
As all vaccination data is being routed via NHS Digital via the import files, once the import process commences, you should not manually add the COVID-19 vaccination data received in emails from national systems.
How will I know when we have received new data?
You are not notified when patient records are updated but you can use Clinical Audit results to monitor vaccination data.
Will the patient's Summary Care Record (SCR) be automatically updated with COVID vaccination data?
Yes. SCRs are automatically updated with COVID vaccination data. However, as you are not logged in with a Smartcard when the import process runs, the summaries are queued. You should follow the usual steps for uploading queued summaries to the Spine on a daily basis as described here.
As you will be aware, the rollout of the COVID-19 vaccination is now taking place across the UK. We have been working closely with each national body and we would like to share with you the following update on our progress and short term implementation plans:
Receiving Vaccinations and Adverse Reaction Data
Federations, Clusters and Primary Care Networks - All Countries
If your Federation/Primary Care Network/Cluster is going to be involved in the national COVID vaccination programme, please can you use one of the national recommended systems for recording COVID vaccinations. This request is based on national guidance issued. This is to allow for consistent data capture and central reporting to take place via existing messaging solutions.
We are working with each national body to receive this data automatically into the patient's record.
Practices
England, Wales & Northern Ireland
It is understood that initially, the majority of COVID-19 immunisations will be administered to the general population via mass vaccine centres in England and Wales. Vaccine and adverse reaction data will be electronically recorded on site using systems selected by national bodies (Pinnacle, Sonar and System C for England and WIS for Wales). The write back of this data to Vision 3 will be implemented in a similar way to the shielded patient lists, whereby Cegedim Healthcare Solutions will be issued with the data from national systems and we will regularly update your patient records with the relevant information.
Scotland
It is understood that initially, the majority of COVID-19 immunisations will be administered to the general population via general practice vaccination clinics and mass vaccine centres in Scotland. Vaccine and adverse reaction data will be electronically recorded on site using systems selected by national bodies. The write back of this data to the patient record is also being managed by a nationally selected third party tool. Please contact your local Health Board with any queries.
All Countries - Vision 3 - Recording Vaccinations
SNOMED version 31.1 has been released to all practices in the UK. This delivers the following new COVID-19 vaccination terms:
-
65F0100 Administration of first dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure)(SNOMED CT Concept 1324681000000101)
-
65F0200 Administration of second dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure) (SNOMED CT Concept 1324691000000104)
We are currently rolling out a further update which allows these codes to be entered within the immunisation Structured Data Area (SDA). This also includes a change to the name in the immunisation type:
-
COVID-19 – Pfizer/BioNTech (previously Courageous)
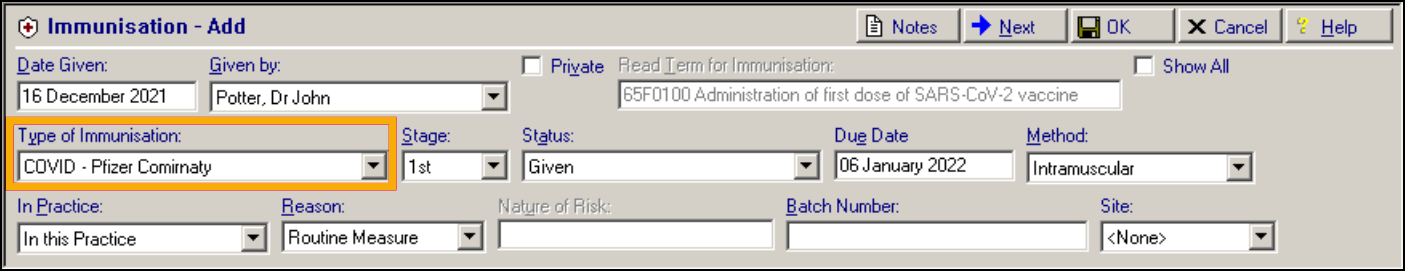
-
COVID-19 – Oxford/AstraZeneca (previously Talent)
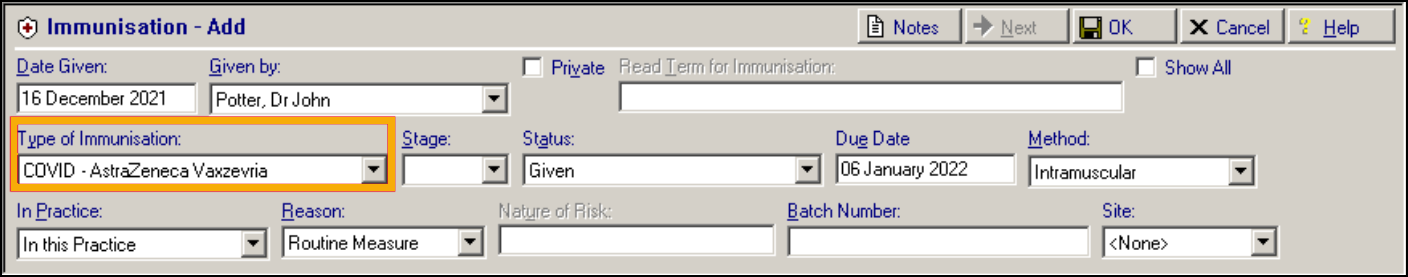
-
COVID-19 – Moderna
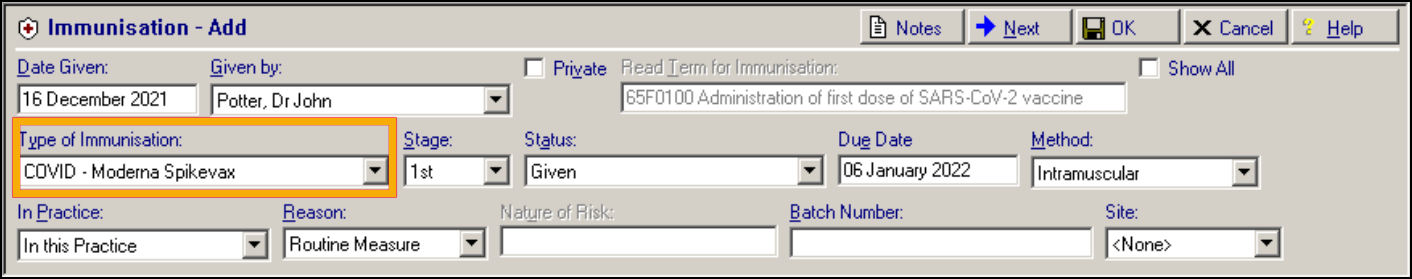
-
COVID-19 – Janssen
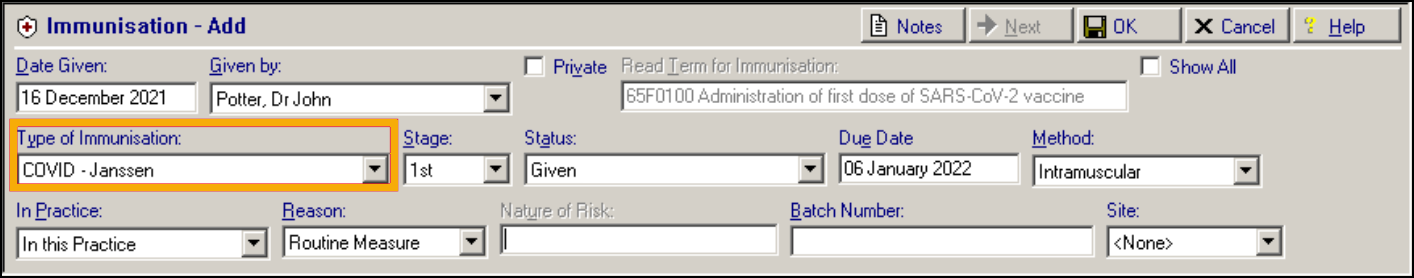
Historical Data Mapping
As part of the update above, we are retrospectively mapping your existing immunisation and medical history records that currently have 65F0.00 2019-nCov (novel coronavirus) vaccination to the equivalent, stage-specific SNOMED/local Read term. This will be done in two phases:
-
Phase 1 - Update to local vaccination code (currently in progress)
-
65F0100 2019-nCoV (novel coronavirus) vaccination
-
65F0200 2019-nCoV (novel coronavirus) vaccination
-
-
Phase 2 - Update to immunisation code description (to be confirmed)
-
65F0100 Administration of first dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure) (SNOMED CT Concept 1324681000000101)
-
65F0200 Administration of second dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure) (SNOMED CT Concept 1324691000000104)
-
Recording vaccinations as drug items
-
November/December Gemscript - These drug dictionaries contain both the Talent and Courageous vaccines which can be recorded as required from your medication screens.
-
January Gemscript - Delivers changes to the Courageous vaccine drug name updating it to "COVID-19 mRNA Vaccine BNT162b2 30micrograms/0.3ml dose concentrate for suspension for injection multidose vials (Pfizer-BioNTech) (Pfizer-BioNTech)".
-
February Gemscript - Delivers changes to the Talent vaccine drug name updating it to "COVID-19 Vaccine AstraZeneca (ChAdOx1 S [recombinant]) 5x10,000,000,000 viral particles/0.5ml dose solution for injection multidose vials (AstraZeneca UK Ltd)".
Recording Adverse Reactions & Allergies
The following COVID-specific terms are available to record adverse reactions to COVID-19 vaccinations in Vision 3 and Vision Anywhere:
-
TJK62 Adverse reaction to severe acute respiratory syndrome coronavirus 2 vaccine
-
14L51 Allergy to severe acute respiratory syndrome coronavirus 2 vaccine
Data Entry Templates & Audits
-
Updated COVID Guideline for Consultation Manager - We have updated the COVID Guideline to include a vaccination management section which allows for quick and easy data display and data entry.
-
Practice Monitoring with Clinical Audit - We have now updated the COVID-19 Clinical Audits to reflect the PRIMIS specification for COVID-19 vaccination uptake.
-
Vision+ - Vision+ release SIS 10570 will contain SNOMED 30 & 31.1 and the December Gemscript drug dictionary updates. This is currently rolling out. We will also be issuing pathway reports to support COVID-19 vaccination management.
The Coronavirus Clinical Audits have been updated:
-
The Coronavirus Administration (COVID-19) audit has been updated. This audit is designed to help you manage the administration of your practice population and those on the shielded patient list.
Click here to download the latest Coronavirus Administration (COVID-19) Clinical Audit.
-
The Coronavirus Vaccination Monitoring audit has been updated. This audit is designed to help you monitor the various priority groups and whether they have or have not yet had a COVID-19 vaccination.
Click here to download the latest Coronavirus Vaccination Clinical Audit.
Click here for instructions on downloading and importing Clinical Audits.
NHS Digital have provided us with the following updated Shielded Patient List (SPL) Letters:
-
To use when informing your patients they have been added to the SPL. We have turned this into a Vision template letter, which can be downloaded from here. (Superseded 26th March 2021)
-
To use when informing your patients they are no longer considered high risk. We have turned this into a Vision template letter, which can be downloaded from here.
As you will be aware, the rollout of the COVID-19 vaccination is now taking place across the UK. We have been working closely with each national body and we would like to share with you the following update on our progress and short term implementation plans:
Receiving Vaccinations and Adverse Reaction Data
Federations, Clusters and Primary Care Networks - All Countries
If your Federation/Primary Care Network/Cluster is going to be involved in the national COVID vaccination programme, please can you use one of the national recommended systems for recording COVID vaccinations. This request is based on national guidance issued. This is to allow for consistent data capture and central reporting to take place via existing messaging solutions.
We are working with each national body to receive this data automatically into the patient's record.
Practices
England, Wales & Northern Ireland
It is understood that initially, the majority of COVID-19 immunisations will be administered to the general population via mass vaccine centres in England and Wales. Vaccine and adverse reaction data will be electronically recorded on site using systems selected by national bodies (Pinnacle, Sonar and System C for England and WIS for Wales). The write back of this data to Vision 3 will be implemented in a similar way to the shielded patient lists, whereby Cegedim Healthcare Solutions will be issued with the data from national systems and we will regularly update your patient records with the relevant information.
Scotland
It is understood that initially, the majority of COVID-19 immunisations will be administered to the general population via general practice vaccination clinics and mass vaccine centres in Scotland. Vaccine and adverse reaction data will be electronically recorded on site using systems selected by national bodies. The write back of this data to the patient record is also being managed by a nationally selected third party tool. Please contact your local Health Board with any queries.
All Countries - Vision 3 - Recording Vaccinations
SNOMED version 31.1 has been released to all practices in the UK. This delivers the following new COVID-19 vaccination terms:
-
65F0100 Administration of first dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure)(SNOMED CT Concept 1324681000000101)
-
65F0200 Administration of second dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure) (SNOMED CT Concept 1324691000000104)
We are currently rolling out a further update which allows these codes to be entered within the immunisation Structured Data Area (SDA). This also includes a change to the name in the immunisation type:
-
COVID-19 – Pfizer/BioNTech (previously Courageous)

-
COVID-19 – Oxford/AstraZeneca (previously Talent)

-
COVID-19 – Moderna

-
COVID-19 – Janssen

Historical Data Mapping
As part of the update above, we are retrospectively mapping your existing immunisation and medical history records that currently have 65F0.00 2019-nCov (novel coronavirus) vaccination to the equivalent, stage-specific SNOMED/local Read term. This will be done in two phases:
-
Phase 1 - Update to local vaccination code (currently in progress)
-
65F0100 2019-nCoV (novel coronavirus) vaccination
-
65F0200 2019-nCoV (novel coronavirus) vaccination
-
-
Phase 2 - Update to immunisation code description (to be confirmed)
-
65F0100 Administration of first dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure) (SNOMED CT Concept 1324681000000101)
-
65F0200 Administration of second dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure) (SNOMED CT Concept 1324691000000104)
-
Recording vaccinations as drug items
-
November/December Gemscript - These drug dictionaries contain both the Talent and Courageous vaccines which can be recorded as required from your medication screens.
-
January Gemscript - Delivers changes to the Courageous vaccine drug name updating it to "COVID-19 mRNA Vaccine BNT162b2 30micrograms/0.3ml dose concentrate for suspension for injection multidose vials (Pfizer-BioNTech) (Pfizer-BioNTech)".
Recording Adverse Reactions & Allergies
The following COVID-specific terms are available to record adverse reactions to COVID-19 vaccinations in Vision 3 and Vision Anywhere:
-
TJK62 Adverse reaction to severe acute respiratory syndrome coronavirus 2 vaccine
-
14L51 Allergy to severe acute respiratory syndrome coronavirus 2 vaccine
Data Entry Templates & Audits
-
Updated COVID Guideline for Consultation Manager - We have updated the COVID Guideline to include a vaccination management section which allows for quick and easy data display and data entry.
-
Practice Monitoring with Clinical Audit - We have now updated the COVID-19 Clinical Audits to reflect the PRIMIS specification for COVID-19 vaccination uptake.
-
Vision+ - Vision+ release SIS 10570 will contain SNOMED 30 & 31.1 and the December Gemscript drug dictionary updates. This is currently rolling out. We will also be issuing pathway reports to support COVID-19 vaccination management.
NHS Digital is providing an additional list of newly identified patients to add to the Shielded Patient List (SPL), which in turn makes them a high priority for COVID-19 vaccinations. These patients have been identified using NHS England's COVID-19 Population Risk Assessment Tool. The purpose of this process is for practices to review the incoming data before it is extracted and sent back to NHS Digital for vaccination call and recall.
The process:
Step 1 - Receiving the data (Automated)
As directed by NHS Digital, we will shortly be adding the following Medical History entry to any Applied or Permanent patients that newly qualify for the 'At Risk' list:
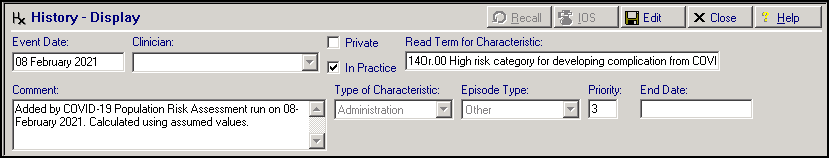
By default the History entry has:
-
Event Date – Date the patient was added to the Shielded Patient List (SPL) as provided by NHS England.
-
Read Term for Characteristic - 14Or.00 High risk category for developing complication from COVID-19 infection.
-
Comment – 'Added by COVID-19 Population Risk Assessment run on <Date>'.
Note - Where a patient has a high risk score calculated using imputed values the comment will also include 'Calculated using assumed values'. -
Priority – 3.
-
End Date – Blank.
-
Consultation Type - Administration.
These new additions should be reviewed by your practice to ensure the high risk category is correctly assigned.
Step 2 - Download the latest COVID -19 Clinical Audits
We have provided a Clinical Audit to help you review the newly identified patients.
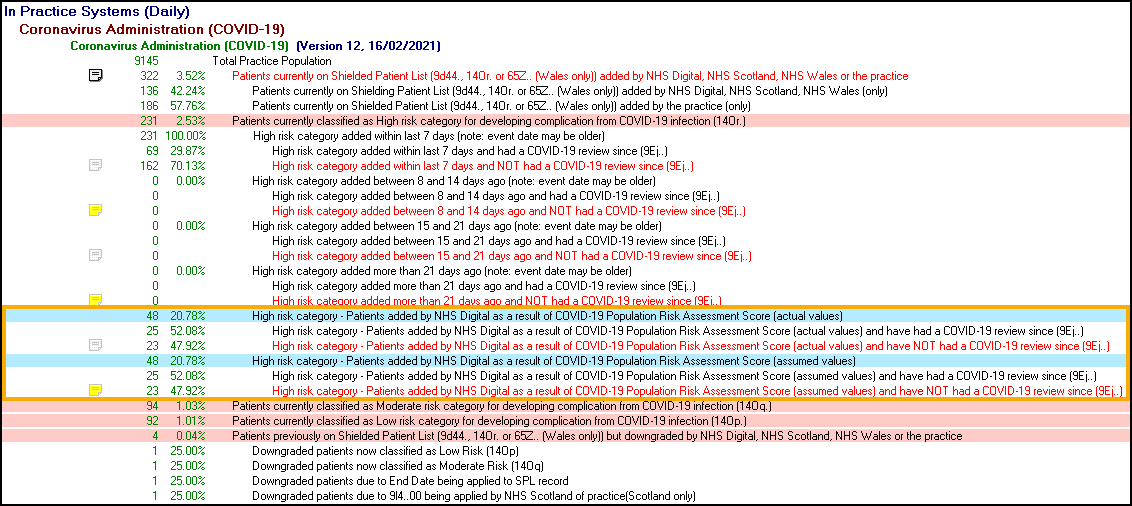
Step 3 - What do I need to do?
To review the newly identified patients that have not been reviewed select the following two audit lines:
-
High risk category - Patients added by NHS Digital as a result of COVID-19 Population Risk Assessment Score (actual values) that have NOT had a COVID-19 review since (9Ej..)
-
High risk category - Patients added by NHS Digital as a result of COVID-19 Population Risk Assessment Score (assumed values) that have NOT had a COVID-19 review since (9Ej..)
Once you have reviewed a patient, see below for what you to do to either, keep patients at high risk or, to downgrade to moderate or low risk:
High Risk Patients
We highly recommend you add an read term of 9Ej..00 Medical records review to the patient record:
-
From Consultation Manager, with the correct patient selected, enter #9Ej in Read Term - Add:
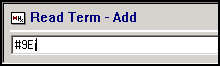
-
Press return and 9Ej..00 Medical records review displays.
-
Select OK.
-
Complete the History - Add screen as required.
- Select OK to save.
Downgrading to Moderate or Low Risk
We highly recommend you add an End date to the high risk entry and then enter a new moderate or low risk entry:
Adding an End Date
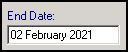
To add an end date to a Medical History:
- Highlight the record, right click and select Edit.
-
Enter the date of the review in End Date
 .
. -
Select OK to save.
Adding a Moderate or Low Risk Entry
To add a moderate or low risk Medical History:
-
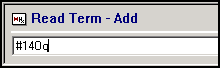
From Consultation Manager, with the correct patient selected, enter one of the following in Read Term - Add:
-
#14Oq, for Moderate risk category for developing complication from COVID-19 infection, or
-
#14Op, for Low risk category for developing complication from COVID-19 infection:
-
-
Press return and then select OK.
-
Complete the History - Add screen as required.
Note - The Event Date should be the same as the End Date on the high risk medical history entry. - Select OK to save.
NHS England have provided a copy of the letter they are sending patients when they are removed from the SPL, click here here for a Vision 3 template of this letter for your use if required.
As you will be aware, the rollout of the COVID-19 vaccination is now taking place across the UK. We have been working closely with each national body and we would like to share with you the following update on our progress and short term implementation plans:
Receiving Vaccinations and Adverse Reaction Data
Federations, Clusters and Primary Care Networks - All Countries
If your Federation/Primary Care Network/Cluster is going to be involved in the national COVID vaccination programme, please can you use one of the national recommended systems for recording COVID vaccinations. This request is based on national guidance issued. This is to allow for consistent data capture and central reporting to take place via existing messaging solutions.
We are working with each national body to receive this data automatically into the patient's record.
Practices
England, Wales & Northern Ireland
It is understood that initially, the majority of COVID-19 immunisations will be administered to the general population via mass vaccine centres in England and Wales. Vaccine and adverse reaction data will be electronically recorded on site using systems selected by national bodies (Pinnacle, Sonar and System C for England and WIS for Wales). The write back of this data to Vision 3 will be implemented in a similar way to the shielded patient lists, whereby Cegedim Healthcare Solutions will be issued with the data from national systems and we will regularly update your patient records with the relevant information.
Scotland
It is understood that initially, the majority of COVID-19 immunisations will be administered to the general population via general practice vaccination clinics and mass vaccine centres in Scotland. Vaccine and adverse reaction data will be electronically recorded on site using systems selected by national bodies. The write back of this data to the patient record is also being managed by a nationally selected third party tool. Please contact your local Health Board with any queries.
All Countries - Vision 3 - Recording Vaccinations
SNOMED version 31.1 has been released to all practices in the UK. This delivers the following new COVID-19 vaccination terms:
-
65F0100 Administration of first dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure)(SNOMED CT Concept 1324681000000101)
-
65F0200 Administration of second dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure) (SNOMED CT Concept 1324691000000104)
We are currently rolling out a further update which allows these codes to be entered within the immunisation Structured Data Area (SDA). This also includes a change to the name in the immunisation type:
-
COVID-19 – Pfizer/BioNTech (previously Courageous)

-
COVID-19 – Oxford/AstraZeneca (previously Talent)

Historical Data Mapping
As part of the update above, we are retrospectively mapping your existing immunisation and medical history records that currently have 65F0.00 2019-nCov (novel coronavirus) vaccination to the equivalent, stage-specific SNOMED/local Read term. This will be done in two phases:
-
Phase 1 - Update to local vaccination code (currently in progress)
-
65F0100 2019-nCoV (novel coronavirus) vaccination
-
65F0200 2019-nCoV (novel coronavirus) vaccination
-
-
Phase 2 - Update to immunisation code description (to be confirmed)
-
65F0100 Administration of first dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure) (SNOMED CT Concept 1324681000000101)
-
65F0200 Administration of second dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure) (SNOMED CT Concept 1324691000000104)
-
Recording vaccinations as drug items
-
November/December Gemscript - These drug dictionaries contain both the Talent and Courageous vaccines which can be recorded as required from your medication screens.
-
January Gemscript - Delivers changes to the Courageous vaccine drug name updating it to "COVID-19 mRNA Vaccine BNT162b2 30micrograms/0.3ml dose concentrate for suspension for injection multidose vials (Pfizer-BioNTech) (Pfizer-BioNTech)".
Recording Adverse Reactions & Allergies
The following COVID-specific terms are available to record adverse reactions to COVID-19 vaccinations in Vision 3 and Vision Anywhere:
-
TJK62 Adverse reaction to severe acute respiratory syndrome coronavirus 2 vaccine
-
14L51 Allergy to severe acute respiratory syndrome coronavirus 2 vaccine
Data Entry Templates & Audits
-
Updated COVID Guideline for Consultation Manager - We have updated the COVID Guideline to include a vaccination management section which allows for quick and easy data display and data entry.
-
Practice Monitoring with Clinical Audit - We have now updated the COVID-19 Clinical Audits to reflect the PRIMIS specification for COVID-19 vaccination uptake.
-
Vision+ - Vision+ release SIS 10570 will contain SNOMED 30 & 31.1 and the December Gemscript drug dictionary updates. This is currently rolling out. We will also be issuing pathway reports to support COVID-19 vaccination management.
As you will be aware, the rollout of the COVID-19 vaccination is now taking place across the UK. We have been working closely with each national body and we would like to share with you the following update on our progress and short term implementation plans:
Federations and Primary Care Networks in England
If your Federation/Primary Care Network is going to be involved in the national COVID vaccination programme, please can you use one of the national recommended systems for recording COVID vaccinations. This request is based on national guidance issued by NHS Digital. This is to allow for consistent data capture and central reporting to take place via existing messaging solutions.
We are working with NHS Digital to receive this data automatically into the patient's GP record and this will then populate the shared care record for Vision patients.
All Countries - Vision 3 - Recording Vaccinations
SNOMED version 31.1 has been released to all practices in the UK. This delivers the following new COVID-19 vaccination terms:
-
65F0100 Administration of first dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure) (SNOMED CT Concept 1324681000000101)
-
65F0200 Administration of second dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure) (SNOMED CT Concept 1324691000000104)
We are currently rolling out a further update which allows these codes to be entered within the immunisation Structured Data Area (SDA). This also includes a change to the name in the immunisation type:
-
COVID-19 – Pfizer/BioNTech (previously Courageous)

-
COVID-19 – Oxford/AstraZeneca (previously Talent)

Historical Data Mapping
As part of the update above, we are retrospectively mapping your existing immunisation and medical history records that currently have 65F0.00 2019-nCov (novel coronavirus) vaccination to the equivalent, stage-specific SNOMED/local Read term. This will be done in two phases:
-
Phase 1 - Update to local vaccination code (currently in progress)
-
65F0100 2019-nCoV (novel coronavirus) vaccination
-
65F0200 2019-nCoV (novel coronavirus) vaccination
-
-
Phase 2 - Update to immunisation code description (to be confirmed)
-
65F0100 Administration of first dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure) (SNOMED CT Concept 1324681000000101)
-
65F0200 Administration of second dose of severe acute respiratory syndrome coronavirus 2 vaccine (procedure) (SNOMED CT Concept 1324691000000104)
-
Recording vaccinations as drug items
-
November/December Gemscript - These drug dictionaries contain both the Talent and Courageous vaccines which can be recorded as required from your medication screens.
-
January Gemscript - Delivers changes to the Courageous vaccine drug name updating it to "COVID-19 mRNA Vaccine BNT162b2 30micrograms/0.3ml dose concentrate for suspension for injection multidose vials (Pfizer-BioNTech) (Pfizer-BioNTech)".
Recording Adverse Reactions & Allergies
The following COVID-specific terms are available to record adverse reactions to COVID-19 vaccinations in Vision 3 and Vision Anywhere:
-
TJK62 Adverse reaction to severe acute respiratory syndrome coronavirus 2 vaccine
-
14L51 Allergy to severe acute respiratory syndrome coronavirus 2 vaccine
Please see Creating a Yellow Card Report from the Drug Allergy and Intolerance screen.
Data Entry Templates & Audits
-
Updated COVID Guideline for Consultation Manager - We have updated the COVID Guideline to include a vaccination management section which allows for quick and easy data display and data entry.
-
Practice Monitoring with Clinical Audit - We have now updated the COVID-19 Clinical Audits to reflect the PRIMIS specification for COVID-19 vaccination uptake.
-
Vision+ - Vision+ release SIS 10570 will contain SNOMED 30 & 31.1 and the December Gemscript drug dictionary updates. This is currently rolling out. We will also be issuing pathway reports to support COVID-19 vaccination management.
Receiving Vaccinations and Adverse Reaction Data
It is understood that initially, the majority of COVID-19 immunisations will be administered to the general population via mass vaccine centres in England and Wales. Vaccine and adverse reaction data will be electronically recorded on site using systems selected by national bodies (Sonar and System C for England and WIS for Wales and Pinnacle). The write back of this data to Vision 3 will be implemented in a similar way to the shielded patient lists, whereby Cegedim Healthcare Solutions will be issued with the data from national systems and we will regularly update your patient records with the relevant information.
It is understood that initially, the majority of COVID-19 immunisations will be administered to the general population via mass vaccine centres in Scotland. Vaccine and adverse reaction data will be electronically recorded on site using systems selected by national bodies. The write back of this data to the patient record is also being managed by a nationally selected third party tool. Please contact your local Health Board with any queries.
As you are aware all adults in England with Down’s Syndrome should be identified and reviewed by practices for inclusion on the Shielded Patient List (SPL).
NHS Digital have updated the Easy Read Down’s Syndrome High Risk Additions patient letter for use when contacting your patients identified with Down’s Syndrome. Select the link below for the updated Vision 3 Template letter:
-
The Coronavirus Vaccination Monitoring audit has been updated. This audit should help you monitor the various priority groups and whether they have or have not yet had a COVID-19 vaccination.
As you will be aware the rollout of the COVID-19 vaccination is rolling out across the UK. We have been working closely with each national body and we would like to share with you the following update on our progress and short term implementation plans:
Federations and Primary Care Networks in England
If your Federation/Primary Care Network is going to be involved in the national COVID vaccination program, please can you use one of the national recommended systems for recording COVID vaccinations. This request is based on national guidance issued by NHS Digital. This is to allow for consistent data capture and central reporting to take place via existing messaging solutions.
We are working with NHS Digital to receive this data automatically into the patients GP record and this will subsequently populate the shared care record for Vision patients.
All Countries
-
Vision 3 - Recording Vaccinations – The generic vaccination term 65F0.00 2019-nCoV (novel coronavirus) vaccination is currently available within Vision 3 and you are able to record the general COVID-19 vaccination term as a medical history entry:
Minor version patches will be released imminently which will allow you to select Courageous (Pfizer-BioNTech), Talent (Oxford University-AstraZenica) or Other Product immunisation types from the Immunisation - Add structured data area which, in turn, records the term 65F0.00 2019-nCoV (novel coronavirus) vaccination:
The SNOMED CT terminology service have also recently issued some new vaccination codes which allow you to select specific terms to record each stage of the vaccine and some other COVID-19 specific clinical terms. This will be released to Vision 3 and Vision Anywhere as part of SNOMED version 31.1 and a further minor version patch with an accompanying immunisation form update. Once you have received these updates, we will retrospectively map your existing immunisation and medical history records to the equivalent, stage-specific SNOMED/local Read term. We will keep you informed as this progresses.
-
Recording vaccinations as drug items – The November Gemscript drug dictionary is currently available to all users and contains both the Talent and Courageous vaccines which can be recorded as required from your medication screens.
-
Recording Adverse Reactions - To record adverse reactions to COVID-19 vaccinations in Vision 3 and Vision Anywhere, from within the allergy form you need to select the drug that the patient has a reaction to from within the allergy form. Normally, this particular type of data entry relies on ingredients of the drug item to be available, which is not the case for COVID-19 vaccines, as such we have made changes to the drug dictionary to allow this to be entered and recognised as an adverse reaction. In short, the ability to record adverse reactions to the COVID-19 vaccines will be available when you have received the December Gemscript drug dictionary. This is due to be deployed imminently.
-
Updated COVID Guideline for Consultation Manager - We have updated the COVID Guideline to include a vaccination management section which allows for quick and easy data display and data entry.
Please download the Guideline from our Consultation Manager Help Centre.Instructions for download and install can also be found here.Note - We will release a similar update to the Vision+ and Vision Anywhere COVID data entry templates imminently. -
Practice Monitoring with Clinical Audit - We have now received the PRIMIS specification for COVID-19 vaccination uptake. We are working on updating the current COVID-19 clinical audits to reflect these requirements. Updates will be posted here.
In the meantime, if required, you can use these existing audits which is based on our interpretation of the latest published Guidance on Priority groups for COVID-19 vaccination. It is largely based on the current PRIMIS Flu specification where possible, or other existing clusters of known codes.
-
Vision+ - Vision+ release SIS 10570 will contain SNOMED 30 & 31.1 and the December Gemscript drug dictionary updates. This is due to be deployed imminently. We will also be issuing pathway reports to support COVID-19 vaccination management.
Receiving Vaccinations and Adverse Reaction Data
It is understood that initially, the majority of COVID-19 immunisations will be administered to the general population via mass vaccine centres in England and Wales. Vaccine and adverse reaction data will be electronically recorded on site using systems selected by national bodies (Sonar and System C for England and WIS for Wales and Pinnacle). The write back of this data to Vision 3 will be implemented in a similar way to the shielded patient lists, whereby Cegedim Healthcare Solutions will be issued with the data from national systems and we will regularly update your patient records with the relevant information.
It is understood that initially, the majority of COVID-19 immunisations will be administered to the general population via mass vaccine centres in Scotland. Vaccine and adverse reaction data will be electronically recorded on site using systems selected by national bodies. The write back of this data to the patient record is also being managed by a nationally selected third party tool. Please contact your local Health Board with any queries.
We are awaiting further information from the BSO on the processing of COVID-19 vaccination and adverse reaction data.
NHS Digital have provided us with an updated Shielded Patient List (SPL) contact letter for you to use when informing your patients they have been added to the SPL. We have turned this into a Vision template letter, which can be downloaded from here.
As you will be aware the rollout of the COVID-19 vaccination is rolling out across the UK. We have been working closely with each national body and we would like to share with you the following update on our progress and short term implementation plans:
All Countries
-
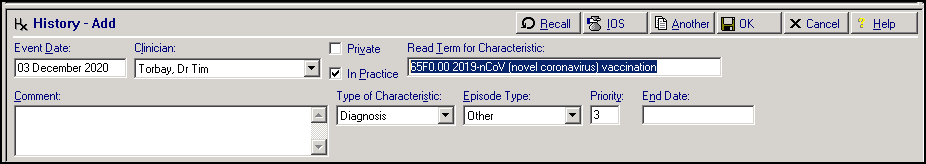
Vision 3 - Recording Vaccinations – The generic vaccination term 65F0.00 2019-nCoV (novel coronavirus) vaccination is currently available within Vision 3 and you are able to record the general COVID-19 vaccination term as a medical history entry:

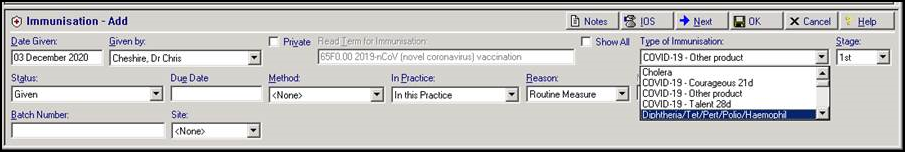
Minor version patches will be released imminently which will allow you to select Courageous (Pfizer-BioNTech), Talent (Oxford University-AstraZenica) or Other Product immunisation types from the Immunisation - Add structured data area which, in turn, records the term 65F0.00 2019-nCoV (novel coronavirus) vaccination:

The SNOMED CT terminology service have also recently issued some new vaccination codes which allow you to select specific terms to record each stage of the vaccine and some other COVID-19 specific clinical terms. This will be released to Vision 3 and Vision Anywhere as part of SNOMED version 31.1 and a further minor version patch with an accompanying immunisation form update. Once you have received these updates, we will retrospectively map your existing immunisation and medical history records to the equivalent, stage-specific SNOMED/local Read term. We will keep you informed as this progresses.
-
Recording vaccinations as drug items – The November Gemscript drug dictionary is currently available to all users and contains both the Talent and Courageous vaccines which can be recorded as required from your medication screens.
-
Recording Adverse Reactions - To record adverse reactions to COVID-19 vaccinations in Vision 3 and Vision Anywhere, from within the allergy form you need to select the drug that the patient has a reaction to from within the allergy form. Normally, this particular type of data entry relies on ingredients of the drug item to be available, which is not the case for COVID-19 vaccines, as such we have made changes to the drug dictionary to allow this to be entered and recognised as an adverse reaction. In short, the ability to record adverse reactions to the COVID-19 vaccines will be available when you have received the December Gemscript drug dictionary. This is due to be deployment in mid-December.
-
Updated COVID Guideline for Consultation Manager - We have updated the COVID Guideline to include a vaccination management section which allows for quick and easy data display and data entry.
Please download the guideline from our Knowledge Base here.Instructions for download and install can also be found here.Note - We will release a similar update to the Vision+ and Vision Anywhere COVID data entry templates imminently. -
Practice Monitoring with Clinical Audit - A suite of Clinical Audits are available to help you monitor vaccine uptake.
-
Vision+ - Vision+ release SIS 10570 will contain SNOMED 30 & 31.1 and the December Gemscript drug dictionary updates. This is due to be deployment in mid-December. We will also be issuing pathway reports to support COVID-19 vaccination management.
Receiving Vaccinations and Adverse Reaction Data
It is understood that initially, the majority of COVID-19 immunisations will be administered to the general population via mass vaccine centres in England and Wales. Vaccine and adverse reaction data will be electronically recorded on site using systems selected by national bodies (Sonar and System C for England and WIS for Wales and Pinnacle). The write back of this data to Vision 3 will be implemented in a similar way to the shielded patient lists, whereby Cegedim Healthcare Solutions will be issued with the data from national systems and we will regularly update your patient records with the relevant information.
It is understood that initially, the majority of COVID-19 immunisations will be administered to the general population via mass vaccine centres in Scotland. Vaccine and adverse reaction data will be electronically recorded on site using systems selected by national bodies. The write back of this data to the patient record is also being managed by a nationally selected third party tool. Please contact your local Health Board with any queries.
We are awaiting further information from the BSO on the processing of COVID-19 vaccination and adverse reaction data.
-
The Coronavirus Vaccination Monitoring audit has been updated. This audit should help you monitor the various priority groups and whether they have or have not yet had a COVID-19 vaccination.
As you will be aware the rollout of the COVID-19 vaccination is imminent across the UK. We have been working closely with each national body and we would like to share with you the following update on our progress and short term implementation plans:
All Countries
-
Vision 3 - Recording Vaccinations – The generic vaccination term 65F0.00 2019-nCoV (novel coronavirus) vaccination is currently available within Vision 3 and you are able to record the general COVID-19 vaccination term as a medical history entry:

Minor version patches will be released imminently which will allow you to select Courageous (Pfizer-BioNTech), Talent (Oxford University-AstraZenica) or Other Product immunisation types from the Immunisation - Add structured data area which, in turn, records the term 65F0.00 2019-nCoV (novel coronavirus) vaccination:

The SNOMED CT terminology service have also recently issued some new vaccination codes which allow you to select specific terms to record each stage of the vaccine and some other COVID-19 specific clinical terms. This will be released to Vision 3 and Vision Anywhere as part of SNOMED version 31.1 and a further minor version patch with an accompanying immunisation form update. Once you have received these updates, we will retrospectively map your existing immunisation and medical history records to the equivalent, stage-specific SNOMED/local Read term. We will keep you informed as this progresses.
-
Recording vaccinations as drug items – The November Gemscript drug dictionary is currently available to all users and contains both the Talent and Courageous vaccines which can be recorded as required from your medication screens.
-
Recording Adverse Reactions - To record adverse reactions to COVID-19 vaccinations in Vision 3 and Vision Anywhere, from within the allergy form you need to select the drug that the patient has a reaction to from within the allergy form. Normally, this particular type of data entry relies on ingredients of the drug item to be available, which is not the case for COVID-19 vaccines, as such we have made changes to the drug dictionary to allow this to be entered and recognised as an adverse reaction. In short, the ability to record adverse reactions to the COVID-19 vaccines will be available when you have received the December Gemscript drug dictionary. This is due to be deployment in mid-December.
-
Practice Monitoring with Clinical Audit - A suite of Clinical Audits are available to help you monitor vaccine uptake.
Click here to download the Coronavirus Vaccination Clinical Audit
Click here for details on Downloading and Importing Clinical Audits
-
Vision+ - Vision+ release SIS 10570 will contain SNOMED 30 & 31.1 and the December Gemscript drug dictionary updates. This is due to be deployment in mid-December. We will also be issuing pathway reports to support COVID-19 vaccination management.
Receiving Vaccinations and Adverse Reaction Data
It is understood that initially, the majority of COVID-19 immunisations will be administered to the general population via mass vaccine centres in England and Wales. Vaccine and adverse reaction data will be electronically recorded on site using systems selected by national bodies (Sonar and System C for England and WIS for Wales and Pinnacle). The write back of this data to Vision 3 will be implemented in a similar way to the shielded patient lists, whereby Cegedim Healthcare Solutions will be issued with the data from national systems and we will regularly update your patient records with the relevant information.
It is understood that initially, the majority of COVID-19 immunisations will be administered to the general population via mass vaccine centres in Scotland. Vaccine and adverse reaction data will be electronically recorded on site using systems selected by national bodies. The write back of this data to the patient record is also being managed by a nationally selected third party tool. Please contact your local Health Board with any queries.
We are awaiting further information from the BSO on the processing of COVID-19 vaccination and adverse reaction data.
As you will be aware the rollout of the COVID-19 vaccination is imminent across the UK. We have been working closely with each national body and we would like to share with you the following update on our progress and short term implementation plans:
All Countries
-
Recording Vaccinations – The generic vaccination term 65F0.00 2019-nCoV (novel coronavirus) vaccination is currently available within Vision 3 and you are able to record the general COVID-19 vaccination term as a medical history entry:

Minor version patches will be released imminently which will allow you to select Courageous (Pfizer-BioNTech), Talent (Oxford University-AstraZenica) or Other Product immunisation types from the Immunisation - Add structured data area which, in turn, records the term 65F0.00 2019-nCoV (novel coronavirus) vaccination:

The SNOMED CT terminology service have also recently issued some new vaccination codes which allow you to select specific terms to record each stage of the vaccine and some other COVID-19 specific clinical terms. This will be released to Vision 3 and Vision Anywhere as part of SNOMED version 31.1 and a further minor version patch with an accompanying immunisation form update. Once you have received these updates, we will retrospectively map your existing immunisation and medical history records to the equivalent, stage-specific SNOMED/local Read term. We will keep you informed as this progresses.
-
Recording vaccinations as drug items – The November Gemscript drug dictionary is currently available to all users and contains both the Talent and Courageous vaccines which can be recorded as required from your medication screens.
-
Recording Adverse Reactions - To record adverse reactions to COVID-19 vaccinations in Vision 3 and Vision Anywhere, from within the allergy form you need to select the drug that the patient has a reaction to from within the allergy form. Normally, this particular type of data entry relies on ingredients of the drug item to be available, which is not the case for COVID-19 vaccines, as such we have made changes to the drug dictionary to allow this to be entered and recognised as an adverse reaction. In short, the ability to record adverse reactions to the COVID-19 vaccines will be available when you have received the December Gemscript drug dictionary. This is due to be deployment in mid-December.
-
Practice Monitoring with Clinical Audit - A suite of Clinical Audits are available to help you monitor vaccine uptake.
Click here to download the Coronavirus Vaccination Clinical Audit
Click here for details on Downloading and Importing Clinical Audits
-
Vision+ - Vision+ release SIS 10570 will contain SNOMED 30 & 31.1 and the December Gemscript drug dictionary updates. This is due to be deployment in mid-December. We will also be issuing pathway reports to support COVID-19 vaccination management.
Receiving Vaccinations and Adverse Reaction Data
It is understood that initially, the majority of COVID-19 immunisations will be administered to the general population via mass vaccine centres in England and Wales. Vaccine and adverse reaction data will be electronically recorded on site using systems selected by national bodies (Sonar and System C for England and WIS for Wales and Pinnacle). The write back of this data to Vision 3 will be implemented in a similar way to the shielded patient lists, whereby Cegedim Healthcare Solutions will be issued with the data from national systems and we will regularly update your patient records with the relevant information.
It is understood that initially, the majority of COVID-19 immunisations will be administered to the general population via mass vaccine centres in Scotland. Vaccine and adverse reaction data will be electronically recorded on site using systems selected by national bodies. The write back of this data to the patient record is also being managed by a nationally selected third party tool. Please contact your local Health Board with any queries.
We are awaiting further information from the BSO on the processing of COVID-19 vaccination and adverse reaction data.
- Adult Patients with Down's Syndrome - All adults in England with Down’s Syndrome should be identified and reviewed by practices for inclusion on the Shielded Patient List (SPL).
NHS Digital have provided us with an updated Shielded Patient letter for your patients who qualify for the SPL and for patients that newly qualify for the SPL based on the above criteria. We have turned these into Vision template letters, which can be downloaded from the following links:
- High risk patients - 201126_Highest_risk_additions_letter_GP.1L.
- Down’s Syndrome Patients:
- Standard - 201126_highest_risk_additions_letter_downs_syndrome_GP.
- Easy Read - 2 December EasyRdletter_downsyndrome_v.0.4.
- Adult Patients with Down's Syndrome - All adults in England with Down’s Syndrome should be identified and reviewed by practices for inclusion on the Shielded Patient List (SPL).
We have updated the Coronavirus clinical audits to support these updates:
- Click here for the latest In Practice Systems Daily audit.
- Click here for details on how to download audits.
NHS Digital have provided us with an updated Shielded Patient letter for your patients who qualify for the SPL and for patients that newly qualify for the SPL based on the above criteria. We have turned these into Vision template letters, which can be downloaded from the following links:
- High risk patients - 201104_Highest_risk_additions_letter_1K.
- Down’s Syndrome Patients:
- Standard - 201104_Highest_risk_additions_letter_Downs_syndrome.
- Easy Read - EasyRdLetter_DownSyndrome_Final 02 11 20.
Pillar 3 SARS-Cov-2 Antibody test results are shortly due to start to flow into Patient Records.
As these come from the same trading partner that Pillar 2 Virus RNA Detection results use, X-Labs National Pathology Exchange (NPEx) no action is required.
All Pillar 3 Antibody Tests look for SARS-Cov-2 IgG antibodies, and results are restricted to Positive, Negative or Unknown.
Vision automatically maps the incoming codes to the SNOMED CT terms below:
|
SNOMED CT Description ID |
SNOMED CT Preferred Term |
Vision Local Code |
|---|---|---|
|
1321541000000108 |
SARS-CoV-2 IgG detected |
43dtA00 |
|
1321571000000102 |
SARS-CoV-2 IgG not detected |
43dtD00 |
|
1321641000000107 |
SARS-CoV-2 IgG detection unkno |
43dtC00 |
NHS Digital have provided us with an updated Shielded Patient letter for you to use for patients who were on the Shielded Patient List (SPL) and now find themselves working in an area affected by local lockdown.
We have turned this into a Vision template letter, which can be downloaded from here
NHS Digital have provided us with:
- An updated Shielded Patient List (SPL) letter for you to use when informing your patients they have been added to the SPL. We have turned this into a Vision template letter, which can be downloaded from here.
- An updated Shielded Patient List (SPL) deduction letter for you to use when informing your patients they have been removed from the SPL. We have turned this into a Vision template letter, which can be downloaded from here.
We have received the first of two lists of Coronavirus/Covid-19 test results reported before the live pathology feed was enabled.
As directed by NHS England, these results were added to your patient records, in the appropriate Viral Studies Structured Data Area (SDA), overnight on 19th June 2020, with the Consultation type of Results Recording.
The Vision local codes for COVID-19 results are:
- 4J3R200 2019-nCoV (novel coronavirus) not detected
- 4J3R400 SARS-CoV-2 detect reslt unknow
- 4J3R100 2019-nCoV (novel coronavirus) detected
The second list is expected to be with us shortly.
For Information Only
We have been advised by NHS Digital that once live results start being received, you may receive more than one test result with the same sample ID for some patient records.
If this happens, the following process applies:
- If the results are the same – The result should be treated as a correct test result.
- If the results are different – Both results require further investigation by NHS 111, and you are not required to do anything.
This only relates to multiple test results with the same sample ID, even if they have different dates.
Individuals will be contacted by NHS 119 where their test results are questionable. No further action is required from you.
This issue is due to users applying the same barcode (Sample ID) to multiple test kits. Over the last week, 3 tests in 300,000 resulted in two different results for one individual, and circa 75 duplicates with the same result.
Changes are underway to prevent this from occurring going forwards.
NWIS have provided us with list of patients that newly qualify for the ‘at risk’ category. Overnight tonight, these patients will have a Medical History entry added to their record to reflect this:
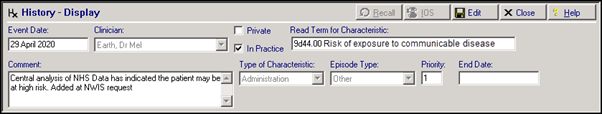
By default the History entry has:
- Read Term for Characteristic - 9d44.00 Risk of exposure to communicable disease
- Comment – Central analysis of NHS Data has indicated the patient may be at high risk. Added at NWIS request
- Priority – 1
These entries will have a Consultation Type of Administration.
It is expected that from the beginning of next week, you will start receiving COVID-19 related pathology messages directly into Mail Manager.
The Vision local codes for COVID-19 results are:
- 4J3R200 2019-nCoV (novel coronavirus) not detected
- 4J3R400 SARS-CoV-2 detect reslt unknow
- 4J3R100 2019-nCoV (novel coronavirus) detected
New results are received into Vision via Mail Manager in the usual way. As previously communicated, because these tests are not initiated by your practice they are not sent to a recognisable clinician. Unless you have set up a Local ID, see Setting up a Local ID below for details, they display in the Unassigned mailbox and require manual allocating and filing in the first instance.
If you have not set up a Local ID in advance, to allocate and file the results to the appropriate patient record:
Setting up a Local ID
- From Mail Manager, select the Staff column, and move to the results sent to *COVIDpillar2, this groups all the COVID-19 results together.
- Depending on how you decide to process these results, you can then either:
- Right click on an individual result, select Allocate to Staff and select a recipient for processing, or
- Tick all the results for one recipient, select Staff
 – Ticked - Allocate to Staff and select a recipient for processing.
– Ticked - Allocate to Staff and select a recipient for processing.
- Once allocated to a clinician for review, the results should be filed to the patient record, tick all the results required and select File
 - File All.
- File All. - Providing the patient is recognised each result files to the Viral Studies SDA in the patient’s record.
- Results can now be reviewed and processed in the usual way.
NHS Digital have provided us with an updated Shielded Patient List (SPL) letter for you to use when informing your patients they have been added to the SPL.
We have turned this into a Vision template letter, which can be downloaded from here.
NWIS have provided us with list of patients that newly qualify for the ‘at risk’ category. Overnight tomorrow tonight, these patients will have a Medical History entry added to their record to reflect this:
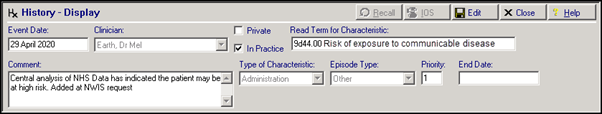
By default the History entry has:
- Read Term for Characteristic - 9d44.00 Risk of exposure to communicable disease
- Comment – Central analysis of NHS Data has indicated the patient may be at high risk. Added at NWIS request
- Priority – 1
These entries will have a Consultation Type of Administration.
Please be aware the Coronavirus (Covid-19) Clinical Audit has been updated, the latest version is Version 9, dated 03/06/2020.
The update includes:
- The top line has been updated to include patients with one of the following records added by NHS Digital/Scotland/Wales, or by yourselves:
- 9d44.00 Risk of exposure to communicable disease
- 65Z..00 Infect.dis.prevent/control NOS, or
- 14Or.00 High risk category for developing complication from COVID-19 infection
that has not been superseded by either:
- 14Oq.00 Moderate risk category for developing complication from COVID-19 infection, or
- 14Op.00 Low risk category for developing complication from COVID-19 infection
- The active reminder against the “Patients currently classified as High risk category for developing complication from COVID-19 infection (14Or.)” line has been removed, as these patients are now included as part of the top line instead.
- There is a new section for patients removed/subtracted from the Shielded Patient List in England. This includes lines to show any mismatch in the downgrading classification between practice and NHS Digital, for example, if one is Low but the other is Moderate.
Click here to update your Clinical Audit as soon as practical
Click here for details on Downloading and Importing Clinical Audits
NHS England have provided us with a list of patients that newly qualify for the ‘at risk’ category, overnight tonight, these patients will have a Medical History entry added to their record to reflect this.
By default the History entry has:
- Event Date – Date the patient was added to the Shielded Patient List (SPL) as provided by NHS England
- Read Term for Characteristic - 14Or.00 High risk category for developing complication from COVID-19 infection
- Comment – Reason provided by NHS England
- Priority – 3
- End Date – Matches Event Date
They will be added with a Consultation Type of Administration.
These patients have already been added to the national SPL and are due to be contacted by NHS England by the end of this week.
NHS England have provided a list of Shielded Patient List (SPL) Subtractions. These are patients that for various reasons are no longer considered ‘high risk’ and now need updating to either ‘moderate’ or ‘low’ risk.
This process consists of two aspects:
- The following entry will be added to any of your patients included in this list:
- Event Date – Will be the date the patient was subtracted from the SPL
- Read Term for Characteristic – Either:
- 14Oq.00 Moderate risk category for developing complication from COVID-19 infection
- 14Op.00 Low risk category for developing complication from COVID-19 infection
- Comment – As per the description provided by NHS England
- Priority – 3
- Consultation Type – Administration
- The original SPL entry, of 14Or.00 High risk category for developing complication from COVID-19 infection, will be updated with an End Date:
- End Date – The date the patient was subtracted from the SPL
The existing Clinical Audit continues to count the entry with the latest Event Date only, so if you have reviewed and updated a patient’s record with a ‘high’, ‘moderate’ or ‘low’ risk entry, these are the records picked up providing the Event Date is after the date received from NHS England.
We are in the process of writing an audit that will identify downgraded records for your review.
NHS England have provided a copy of the letter they are sending patients when they are removed from the SPL, click here for a Vision template of this letter for your use if required.
There is a dedicated Yellow Card site for the reporting of new and emerging side effects and medical device incidents in COVID-19 treatments.
This includes the reporting of side effects for medicines taken by patients to manage long-term or pre-existing conditions. Please report any new and emerging side effects and medical device incidents in COVID-19 treatments here:
- https://coronavirus-yellowcard.mhra.gov.uk/
It is important you do not use the integrated Yellow Card functionality within Vision 3 for the reporting of the side effects and medical device incidents in COVID-19 treatments.
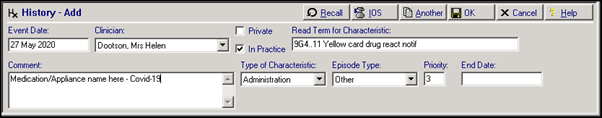
The following entry could be used to record the Yellow Card report in the patient’s clinical record:
- Read Term for Characteristic – 9G4..11 Yellow card drug react notif
- Comment - Medication/Appliance name – COVID-19
NHS England have provided us with a list of patients that newly qualify for the ‘at risk’ category, overnight tonight, these patients will have a Medical History entry added to their record to reflect this.
Important – We have been directed by NHS England to include the provided reason in Comments for these entries. This can contain sensitive information. For this reason the new entry is being added as a Priority 3 to give you time to review the entry without it automatically being loaded to the SCR when accessed.
By default the History entry has:
Event Date – Date the patient was added to the Shielded Patient List (SPL) as provided by NHS England
Read Term for Characteristic - 14Or.00 High risk category for developing complication from COVID-19 infection
Comment – Reason provided by NHS England
Priority – 3
End Date – Matches Event Date
They will be added with a Consultation Type of Administration.
These patients have already been added to the national SPL and are due to be contacted by NHS England by the end of this week.
NWIS have provided us with list of patients that newly qualify for the ‘at risk’ category. Overnight tonight, these patients will have a Medical History entry added to their record to reflect this:
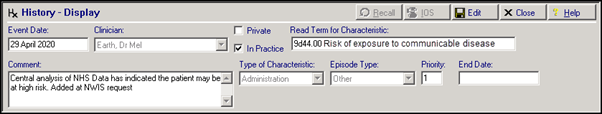
By default the History entry has:
- Read Term for Characteristic - 9d44.00 Risk of exposure to communicable disease
- Comment – Central analysis of NHS Data has indicated the patient may be at high risk. Added at NWIS request
- Priority – 1
These entries will have a Consultation Type of Administration.
You will shortly be receiving the Coronavirus test results from the swabs taken at the nationwide Coronavirus test centres.
These results are received in the same way as normal pathology results, however as the tests were not requested by yourselves, they are sent to a generic recipient at your practice:
- GP Code – G9999981
- GP name - COVIDpillar2.
To ensure these results automatically file to the patient record on receipt, we strongly recommend you set up a Local ID for G9999981.
To set up a Local ID
- From Mail Manager , select Tools - Local IDs.
- The Local IDs screen displays:
- Select Add and the Add a new Local Identifier screen displays:
- Complete as required:
- Local Identifier - Enter G9999981
- Staff Member - Select the appropriate member of staff from the available list
- Select OK.
NHS England have provided us with a list of patients that newly qualify for the ‘at risk’ category, overnight tonight, these patients will have a Medical History entry added to their record to reflect this.
By default the History entry has:
- Event Date – Date the patient was added to the Shielded Patient List (SPL) as provided by NHS England
- Read Term for Characteristic - 14Or.00 High risk category for developing complication from COVID-19 infection
- Comment – Reason provided by NHS England
- Priority – 3
- End Date – Matches Event Date
They will be added with a Consultation Type of Administration.
These patients have already been added to the national SPL and will be contacted by NHS England.
NWIS have provided us with list of patients that newly qualify for the ‘at risk’ category. Overnight tonight, these patients will have a Medical History entry added to their record to reflect this:
By default the History entry has:
- Read Term for Characteristic - 9d44.00 Risk of exposure to communicable disease
- Comment – Central analysis of NHS Data has indicated the patient may be at high risk. Added at NWIS request
- Priority – 1
These entries will have a Consultation Type of Administration.
NHS England have provided us with a list of patients that newly qualify for the ‘at risk’ category. Overnight tonight, these patients will have a Medical History entry added to their record to reflect this.
By default the History entry will have:
- Event Date – Date the patient was added to the Shielded Patient List (SPL) as provided by NHS England
- Read Term for Characteristic - 14Or.00 High risk category for developing complication from COVID-19 infection
- Comment – Reason provided by NHS England
- Priority – 3
- End Date – Matches Event Date
They will be added with a Consultation Type of Administration.
These patients have already been added to the national SPL and are due to be contacted by NHS England.
NHS England has issued a new letter for you to send to your ‘at risk’ patients, we have created a version with the correct Vision merge fields for you to download from here.
NWIS have provided us with list of patients that newly qualify for the ‘at risk’ category. Overnight last night, these patients had a Medical History entry added to their record to reflect this.
By default the History entry has:
Read Term for Characteristic - 9d44.00 Risk of exposure to communicable disease
Comment – Central analysis of NHS Data has indicated the patient may be at high risk. Added at NWIS request
Priority – 1
They were added with a Consultation Type of Administration.
NHS England have provided us with a list of patients that newly qualify for the ‘at risk’ category, in the next few days, these patients will have a Medical History entry added to their record to reflect this:
By default the History entry has:
- Event Date – Date the patient was added to the Shielded Patient List (SPL) as provided by NHS England
- Read Term for Characteristic - 14Or.00 High risk category for developing complication from COVID-19 infection
- Comment – Reason provided by NHS England
- Priority – 3
They will be added with a Consultation Type of Administration.
These patients have already been added to the national Shielded Patient List and are due to be contacted by NHS England by the end of this week.
What do you need to do?
We are currently working on an update of the Coronavirus/COVID-19 Clinical Audit which will be available to download shortly.
This audit will highlight any patients with one of the following codes on their record, but without 9Ej..00 Medical records review recorded since:
- 14Op.00 - Low risk category for developing complication from COVID-19 infection
- 14Oq.00 - Moderate risk category for developing complication from COVID-19 infection
- 14Or.00 - High risk category for developing complication from COVID-19 infection
This should highlight all those patients still requiring review.
We have been asked to remind you:
Any patients you locally identified as clinically extremely vulnerable prior to 28 April should now be recognised by the Government support website.
You should have written to all of these patients, using the standard letter previously provided. If you have not done so, please ensure that you write to these patients as soon as possible. Please use this updated version of the letter. This contains the same information but confirms that the Government is currently advising people who are clinically extremely vulnerable to shield until 30 June, subject to ongoing review.
People who you have identified locally and added to the registry will be sent text messages later this week and contacted early next week by the Government support service call centre if they have not yet registered online or by phone. It is therefore critical that you have contacted them to confirm that they have been identified as clinically extremely vulnerable.
We are aware that some people who believe they have registered for support on the website have not received it. We have fed this back to the Government service and are working with them to resolve issues with the website and call centre. Please advise people to re-register ensuring they have entered the correct NHS number on the website and their name and address as used in their NHS records.
We have received a number of queries about the length of time patients should shield for. The Government is currently advising people to shield until at least the 30 June (irrespective of when the letter from the NHS was received) and is regularly monitoring this position. Further information about shielding will be published in due course by Government and individuals currently shielding notified.
As a reminder, the websites below may contain helpful information about these patients/processes:
- Highest clinical risk being advised to shield identification methodology – https://digital.nhs.uk/coronavirus/shielded-patient-list/methodology
- Shielded patient list guidance for general practice – https://digital.nhs.uk/coronavirus/shielded-patient-list/guidance-for-general-practice
- Government’s shielding guidance – https://www.gov.uk/government/publications/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19
As directed by NHS Digital, for all your active patients with a clinical entry of 9d44.00 recorded since 1st January 2020 without an End Date added, we are running a utility to add the following clinical record:
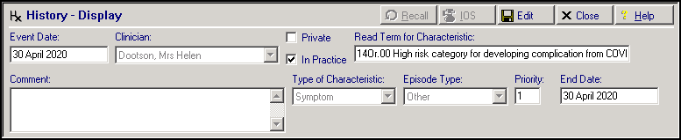
By default the History entry will have:
- Read Term for Characteristic - 14Or.00 High risk category for developing complication from COVID-19 infection
- Comment – Blank
- Priority – 1
- End Date – Matches Event Date
They will be added with a Consultation Type of Administration.
NWIS have asked us to update all the entries made to your original list of patients that qualify for the ‘at risk’ category.
In the next few days we will run an overnight utility changing the Consultation Type of those entries to Administration.
Did you know, the NHS has provided a widget for you to add to your practice website?
This can provide your patients the latest coronavirus information as soon as it is available.
To see details of the widget and instructions for adding it to your website, click here https://developer.api.nhs.uk/coronavirus/widget
NWIS have provided us with a list of patients that newly qualify for the ‘at risk’ category, overnight tonight, these patients will have a Medical History entry added to their record to reflect this.
By default the History entry will have:
- Read Term for Characteristic - 9d44.00 Risk of exposure to communicable disease
- Comment – Central analysis of NHS Data has indicated the patient may be at high risk. Added at NWIS request
- Priority – 1
They will be added with a Consultation Type of Administration.
For practices in England, when you receive an NHS111 message into Mail Manager which contains one of the Covid-19 disposition codes listed below, Vision now automatically generates and files a Medical History entry onto the patient record with the following details:
- Event Date – The date of the contact
- Read Term for Characteristic – 1JX1.00 Suspected disease caused by 2019-nCoV (novel coronavirus)
- Comment – 111 Disposition DXnnnn with the Dx code and description from the NHS111 message (see below)
- Type of Characteristic – Symptom
- Episode Type – Other
- Priority - 1
- Consultation Type – NHS Direct Report
The Disposition Codes which automatically file are:
- Dx1112 - COVID risk Clinical Assessment service 1 hour
- Dx1113 - COVID risk Clinical Assessment service 2 hours
- Dx1114 - COVID risk Clinical Assessment service 4 hours
- Dx1115 - COVID risk Clinical Assessment service 6 hours
- Dx1116 - COVID risk Clinical Assessment service 12 hours
- Dx451 - COVID Coordination Service
- Dx391 - COVID Self Care
- Dx01125 - Emergency Ambulance Response for Potential COVID19
- Dx339 - Speak to a Clinician from our service Immediately – COVID 19 Chest Pain Assessment
- DX01214 - Emergency Ambulance Response for Potential COVID19 (Category 3)
NHS Digital have provided us with a list of patients who have self-declared as ‘at risk’ on the Public Health England web site.
NHS Digital has asked us to add the following record to these patients:
- In Practice – Tick removed
- Read Term for Characteristic - 9N38.00 Message from patient
- Comment – ‘Added by Cegedim Health Systems on behalf of NHS Digital to flag patients self-declaring as in the high risk category for developing complications from Covid-19 infection’
- Priority – 3
- Consultation Type - Administration
This utility will be run on your system tonight.
NHS Digital have issued guidance on the processing of these patients for your reference here.
This guidance includes the templates of the two letters they are asking you to send to the two groups of patients:
- One for those reviewed and deemed high risk, the template of which was sent to you by NHS Digital in March, click here for the Word template
- One for those reviewed and considered moderate or low risk, click here for the Word template
Please be aware the Coronavirus (Covid-19) Clinical Audit has just been updated, the latest version is Version 7, dated 27/4/2020.
The update includes:
- Clinical terms 9d44.00 and 65Z..00 are now split between:
- 9d44.00 and 65Z..00 records without an End date entered in the Medical History record, and
- 9d44.00 and 65Z..00 records with an End date entered in the Medical History record.
This allows for patients to be excluded from the COVID-19 list for developing complications if required.
- An update to the clinical term 9N38.00 lines, to include those with a free text of ‘COVID-19: Patient self-declared online as extremely vulnerable - requires clinical review’ only
- Patients coded with Shielded Patient risks (based on English GPES extract specifications) now has FOR INFORMATION ONLY added to it and the following lines updated:
- Solid organ transplant recipients now included all appropriate patients
- Severe respiratory conditions now excludes inappropriate terms, for example, Flu-like symptoms and Upper and Lower respiratory tract infection
Click here to update your Clinical Audit as soon as practical
Click here for details on Downloading and Importing Clinical Audits
Please be aware the Coronavirus (Covid-19) Clinical Audit has just been updated, the latest version is Version 6, dated 21/4/2020.
The update includes:
- Re-order - A re-order of the audit lines to ensure the most important line is at the top
- For England:
- New lines for the High/Moderate/Low risk codes available in the April 2020 SNOMED CT release, see SNOMED CT Changes and New Vision Local Codes April 2020 for details
- New lines for patients with self-declared code Message from patient with ‘COVID-19: Patient self-declared online as extremely vulnerable – requires clinical review’ in the comments. An active Reminder has been added for those not yet reviewed by your practice:
- The NHS111 section has an active reminder that stays active for one month:
- A new section replicating the GPES audit identifying the highest risk patients, but using the Vision data. There are some additional lines to help identify patients who are in or missing from the NHS list or the Vision generated Shielded list
- For Scotland:
- New lines for Key Information Summary (KIS) support
- For Wales:
- New lines to highlight patients with 65Z..00 Infect.dis.prevent/control NOS recorded
Click here to update your Clinical Audit as soon as practical
Click here for details on Downloading and Importing Clinical Audit
As directed by NHS Scotland, any entries created by the utility run on your patient register to highlight patients in the ‘most vulnerable’ category for developing complications from Covid-19 infection, will in the next few days:
- Have the Consultation Type they were recorded under updated to Administration
Any patients that newly qualify for the ‘most vulnerable’ category and that are included in the latest download from NHS Scotland, will have a Medical History entry added to their record to reflect this.
If you have manually removed the 9d44.00 entry added as part of one of the previous NHS Scotland directed updates, it is not reinstated unless the patient is included as a new entry on the latest list from NHS Scotland.
As directed by NHS Digital, any entries created by the utility run on your patient register on the 23rd March to highlight patients in the ‘at risk’ category for developing complications from Covid-19 infection, will in the next few days:
- Have that Medical History entry updated from a priority 3 to a priority 1 ensuring the entry is updated to the patient’s Summary Care Record without further interaction from yourselves. See SCR in Consultation Manager for further details.
- Have a reason for inclusion added to any existing Comments
- Where a reason for inclusion exists – ‘Central analysis of NHS Data has indicated the patient may have these risks:…’ and the reason(s) for inclusion listed
- Where a reason for inclusion is not included in the download from NHS Digital - Central analysis of NHS Data has indicated the patient may be at high risk’
Any patients that newly qualify for the ‘at risk’ category and that are included in the latest download from NHS Digital, will have a Medical History entry added to their record to reflect this.
As previously reported, NHS England have written to 900,000 at very high risk patients advising them to stay indoors (shield) for the next 12 weeks.
As directed by NHS Digital, overnight on 23 March, a utility was run on your patient records and any patients falling in the ‘at highest risk’ group as specified by NHS Digital now have the Read code 9d44.00 Risk of exposure to communicable disease (situation) added to their record.
By default the associated History entry for patients identified as at-risk contains the following details:
- Read Term for Characteristic - 9d44.00 Risk of exposure to communicable disease (situation) - Note the SNOMED CT Description is used for the terms automatically added to patient records.
- Comment - High risk category for developing complications from Covid-19 infection - Added at NHS Digital request.
- Priority – 3.
At-Risk Patients – Phase 2
NHS England have identified additional At-Risk patients as part of phase 2 of this process. This list also includes the reason why the patient has been included in the list. NHS Digital have only just released this list to us (9th April 2020) to allow us to update the patients at your practice. We are working to create and run another utility which will add the clinical term "9d44.00 Risk of exposure to communicable disease (situation)" to the appropriate patient records. The reason why the patient has been identified as at highest risk will also be included in the free text comments.
As part of phase 2, we will also ensure that all at highest risk clinical terms are flagged as priority 1 medical history. This means they will be included in SCR uploads for consenting patients.
Once the utility has been run, you can review the newly added patients via the Clinical Audit, as detailed below, and once you have received the April 2020 SNOMED CT update, due to start release from 15 April 2020, you can use the following clinical terms to classify the patient risk:
- 14Op.00 Low risk category for developing complication from COVID-19 infection
- 14Oq.00 Moderate risk category for developing complication from COVID-19 infection
- 14Or.00 High risk category for developing complication from COVID-19 infection
Manually Recording a Patient is At Highest Risk.
If you have any patients in this vulnerable category that have not been identified with the term 9d44.00 for any reason, for example, newly registered patients, patients with new at risk diagnoses or patients prescribed drugs by a hospital, Read term 9d44.00 Potential infectious contact must be manually added to their clinical record with a Priority of 1 and "High risk category for developing complications from Covid-19" entered into free text comments.
Reviewing At Highest Risk Patients
You can view the patients who have had the 9d44.00 clinical term added to their record by downloading and importing the latest Coronavirus Clinical Audit from here and reviewing the Patients flagged by NHS Digital as increased risk for developing complications from Covid-19 infection (9d44) group:
The group has an active Reminder of ‘At significant increased risk from COVID-19’.
Dr Tom Hodges-Hoyland
Medical Adviser/Clinical Safety Officer
Vision
See Downloading and Importing Clinical Audits for details on downloading and importing Clinical Audits.
As extensively reported, NHS Scotland will be writing to your ‘most vulnerable’ patients advising them to stay indoors for the next 12 weeks.
As directed by NHS Scotland, we have run a utility on your patient records and any patients falling in the ‘at risk’ group as specified by NHS Scotland now have clinical term 9d44.00 Risk of exposure to communicable disease (situation) added to their record.
By default the associated History entry has:
- Read Term for Characteristic - 9d44.00 Potential infectious contact - note the SNOMED CT Description is used for the terms automatically added to patient records.
- Comment - High risk category for developing complications from Covid-19 - Added at NSS request.
- Priority – 1, meaning it is included in Emergency Care Summary (ECS) and Key Information Summary uploads
If you have any patients in this vulnerable category that have not been identified with the term 9d44.00 for any reason, for example, newly registered patients, patients with new at risk diagnoses or patients prescribed drugs by a hospital, Read term 9d44.00 Potential infectious contact must be manually added to their clinical record with a Priority of 1 and High risk category for developing complications from Covid-19 entered into Comments.
Viewing your Coronavirus/Covid-19 ‘Most Vulnerable’ Patients
You can view the patients who have had the 9d44.00 clinical term added to their record by downloading and importing the latest Coronavirus Clinical Audit from here https://help.visionhealth.co.uk/clinical%20audit/Content/Downloads/INPS%20Daily.htm and reviewing the Patients flagged by NHS Scotland as increased risk for developing complications from Covid-19 infection (9d44) group:
The group has an active Reminder of ‘At significant increased risk from COVID-19’.
See Downloading and Importing Clinical Audits for details on downloading and importing Clinical Audits.
As directed by NHS Digital, any entries created by the utility run on your patient register on the 23rd March to highlight patients in the ‘at risk’ category for developing complications from Covid-19 infection, will in the next few days:
- Have that Medical History entry updated from a priority 3 to a priority 1 ensuring the entry is updated to the patient’s Summary Care Record without further interaction from yourselves. See SCR in Consultation Manager for further details.
- Have a reason for inclusion added to any existing Comments:
- Where a reason for inclusion exists – ‘Central analysis of NHS Data has indicated the patient may have these risks:…’ and the reason(s) for inclusion listed
- Where a reason for inclusion is not included in the download from NHS Digital - Central analysis of NHS Data has indicated the patient may be at high risk’
Any patients that newly qualify for the ‘at risk’ category and that are included in the latest download from NHS Digital, will have a Medical History entry added to their record to reflect this:
As extensively reported, NHS Wales will be writing to your ‘most vulnerable’ patients advising them to stay indoors for the next 12 weeks.
As directed by NHS Wales, we have run a utility on your patient records and any patients falling in the ‘at risk’ group as specified by NHS Wales now have clinical term 9d44.00 Risk of exposure to communicable disease (situation) added to their record.
By default the associated History entry has:
- Read Term for Characteristic - 9d44.00 Risk of exposure to communicable disease (situation) - note the SNOMED CT Description is used for the terms automatically added to patient records.
- Comment - High risk category for developing complications from Covid-19 infection - Added at NWIS request.
- Priority – 1, meaning it is included in WCCG data.
If you have any patients in this vulnerable category that have not been identified with the term 9d44.00 for any reason, for example, newly registered patients, patients with new at risk diagnoses or patients prescribed drugs by a hospital, Read term 9d44.00 Potential infectious contact must be manually added to their clinical record with a Priority of 1 and High risk category for developing complications from Covid-19 entered into Comments.
Viewing your Coronavirus/Covid-19 ‘Most Vulnerable’ Patients
You can view the patients who have had the 9d44.00 clinical term added to their record by downloading and importing the latest Coronavirus Clinical Audit from here https://help.visionhealth.co.uk/clinical%20audit/Content/Downloads/INPS%20Daily.htm and reviewing the Patients flagged by NHS Scotland as increased risk for developing complications from Covid-19 infection (9d44) group:
The group has an active Reminder of ‘At significant increased risk from COVID-19’.
See Downloading and Importing Clinical Audits for details on downloading and importing Clinical Audits.
Please click here to visit the NHS England website for Guidance and updates for GPs: Highest clinical risk patients. NHS England's page includes information and advice on the following:
- Update on the Government’s shielding policy and implications for General Practice: 3 April 2020
- Caring for people at highest clinical risk from COVID-19: 3 April 2020
We have issued a new Guideline to support you in caring for your patients.
Click here to download the latest Coronavirus/Covid 19 Guideline
We are now in the process of releasing new clinical terms to support the recording of Coronavirus across the Vision product suite.
Vision 3
This update is being released on top of the following versions of Vision 3:
- England – Vison release DLM 700 - Complete as of 5th March 2020
- Scotland – Vision release DLM 650 - Almost complete as of 5th March 2020
- Wales – Vision release DLM 660 - Complete as of 5th March 2020
- Northern Ireland – Vision release 670 - In progress
As you may be aware, Vision 3 dual codes all data, data is entered using Read codes and mapped to SNOMED CT behind the scenes. From April 2018 there are additions to SNOMED CT that cannot not be directly mapped to existing Read codes. These can be recorded in Vision Anywhere, which is fully SNOMED CT compliant.
This change report lists the Vision local codes we are introducing to ensure you can utilise the new SNOMED CT codes that cannot be directly mapped to Read codes within Vision 3.
Clinical Audit Support
To support you in your management of Coronavirus (COVID-19) we have produced a suite of audits for you to download and use:
This audit can be downloaded from https://help.visionhealth.co.uk/clinical%20audit/Content/Downloads/INPS%20Daily.htm
For instructions on downloading and importing audits see Downloading and Importing Clinical Audits
Vision +
Please be aware, the new Coronavirus clinical terms are not available in Vision + until you receive SIS10500, due for release in the near future.
Vision Anywhere
The new clinical terms to support the recording of Coronavirus are due to be released as soon as Vision 3 has been updated to receive and map the SNOMED CT terms.
Click here for the Coronavirus Clinical Term Update - February 2020 release guide
Following requests from national bodies, the Patient Services (England, Scotland and Northern Ireland) and My Health Online (Wales) patient login screens have been updated to display the below advice regarding coronavirus:
England, Northern Ireland & Scotland
----------------------------------------------------------------------
Coronavirus (COVID-19)
Get information about coronavirus on NHS.UK
Do not book a GP appointment if you think you might have coronavirus.
Stay at home and avoid close contact with other people.
Use the 111 coronavirus service to see if you need medical help
----------------------------------------------------------------------
Click here to view the Patient Services Website
My Health Online in Wales
Do not book a GP appointment if you think you might have coronavirus.
Stay at home and avoid close contact with other people.
Use the 111 coronavirus service to see if you need medical help
Further information available here.
This message is also displayed in Welsh:
Please be aware the Coronavirus (Covid-19) Clinical Audit has just been updated due to notification of a code change from NHS Digital, the latest version is Version 4, dated 25/3/2020.
NHS111 messages with the disposition code of Dx020 Ambulance for Unexpected Death, Under 18 are no longer included in the NHS111 Covid-19 messages groups.
If you are processing your NHS111 Covid-19 messages using the Clinical Audit, you should not add the 1JX1.00 - Suspected disease caused by 2019-nCoV (novel coronavirus) clinical code to the patient record.
Click here to update your Clinical Audit as soon as practical
Click here for details on Downloading and Importing Clinical Audits